Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Mass Spectrometry Determining the molecular formula of an unknown compound Model 1: Mass Spectrum of Acetone Questions 1. Label the peak due to the molecular
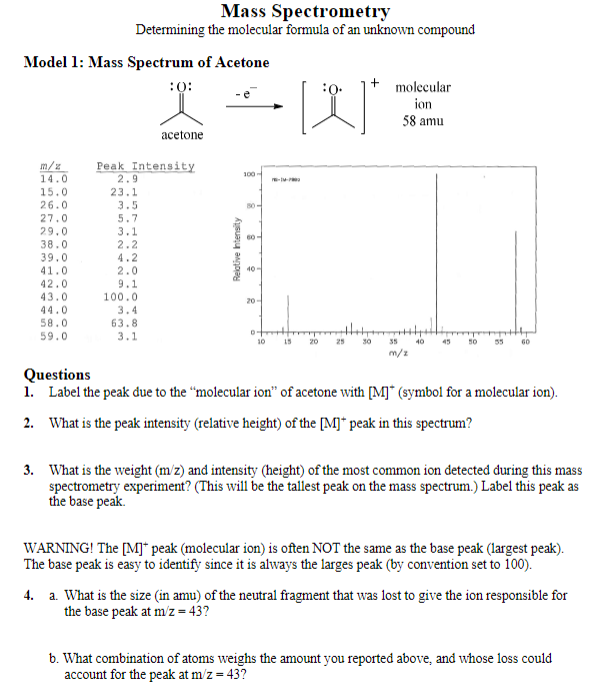

Mass Spectrometry Determining the molecular formula of an unknown compound Model 1: Mass Spectrum of Acetone Questions 1. Label the peak due to the "molecular ion" of acetone with [M] (symbol for a molecular ion). 2. What is the peak intensity (relative height) of the [M]+peak in this spectrum? 3. What is the weight (m/z) and intensity (height) of the most common ion detected during this mass spectrometry experiment? (This will be the tallest peak on the mass spectrum.) Label this peak as the base peak. WARNING! The [M]+peak (molecular ion) is often NOT the same as the base peak (largest peak). The base peak is easy to identify since it is always the larges peak (by convention set to 100). 4. a. What is the size (in amu) of the neutral fragment that was lost to give the ion responsible for the base peak at m/z=43 ? b. What combination of atoms weighs the amount you reported above, and whose loss could account for the peak at m/z=43 ? The major peaks on a mass spectrum representing ions lighter than the molecular ion are called fragment peaks. Draw an ion that could account for the m/z=43 peak
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


