Answered step by step
Verified Expert Solution
Question
1 Approved Answer
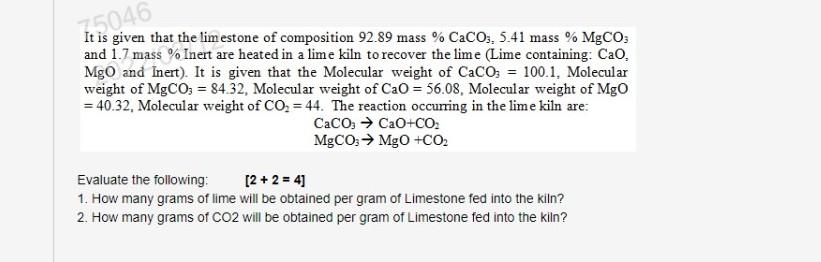
need answer with steps 75046 It is given that the limestone of composition 92.89 mass % CaCO3, 5.41 mass % MgCO: and 1.7 mass %
need answer with steps
75046 It is given that the limestone of composition 92.89 mass % CaCO3, 5.41 mass % MgCO: and 1.7 mass % Inert are heated in a lime kiln to recover the lime (Lime containing: Ca. MgO and Inert). It is given that the Molecular weight of CaCO3 = 100.1, Molecular weight of MgCO3 = 84.32. Molecular weight of Cao = 56.08, Molecular weight of Mgo = 40.32, Molecular weight of CO2 = 44. The reaction occurring in the lime kiln are: CaCO3 + CaO+CO: MgCO: MgO +CO Evaluate the following: [2 + 2 = 4) 1. How many grams of lime will be obtained per gram of Limestone fed into the kiln? 2. How many grams of CO2 will be obtained per gram of Limestone fed into the kilnStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


