Question
One mole of a monatomic ideal gas is taken on the path A B C D A, as shown in Figure. All paths are reversible.
One mole of a monatomic ideal gas is taken on the path A B C D A, as shown in Figure. All paths are reversible.
A B is a reversible isothermal expansion of the gas.
B C is a reversible adiabatic expansion of the gas.
C D is a reversible isothermal compression of the gas.
D A is a reversible adiabatic compression of the gas.
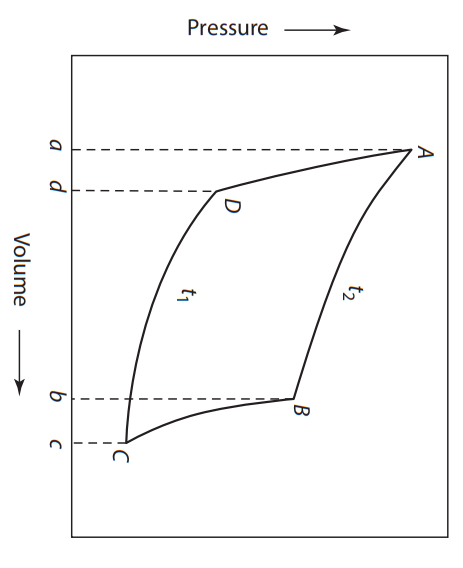
Figure: A cycle for one mole of a monatomic ideal gas with two isothermal paths and two adiabatic paths. The area enclosed by ABCD is the work done by the gas during the cycle.
a. Derive expressions for U, q, and w during each step in terms of Va , Vb , Vc , Vd , t1, t2, and R. Determine the sign of each.
b. Determine the values of (wi), (qi), and (Ui) in terms of Va , Vb , Vc , Vd , t1, t2, and R. Determine the sign of each.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


