Answered step by step
Verified Expert Solution
Question
1 Approved Answer
One mole of iron (6 1023 atoms) has a mass of 56 grams, and its density is 7.87 grams per cubic centimeter, so the
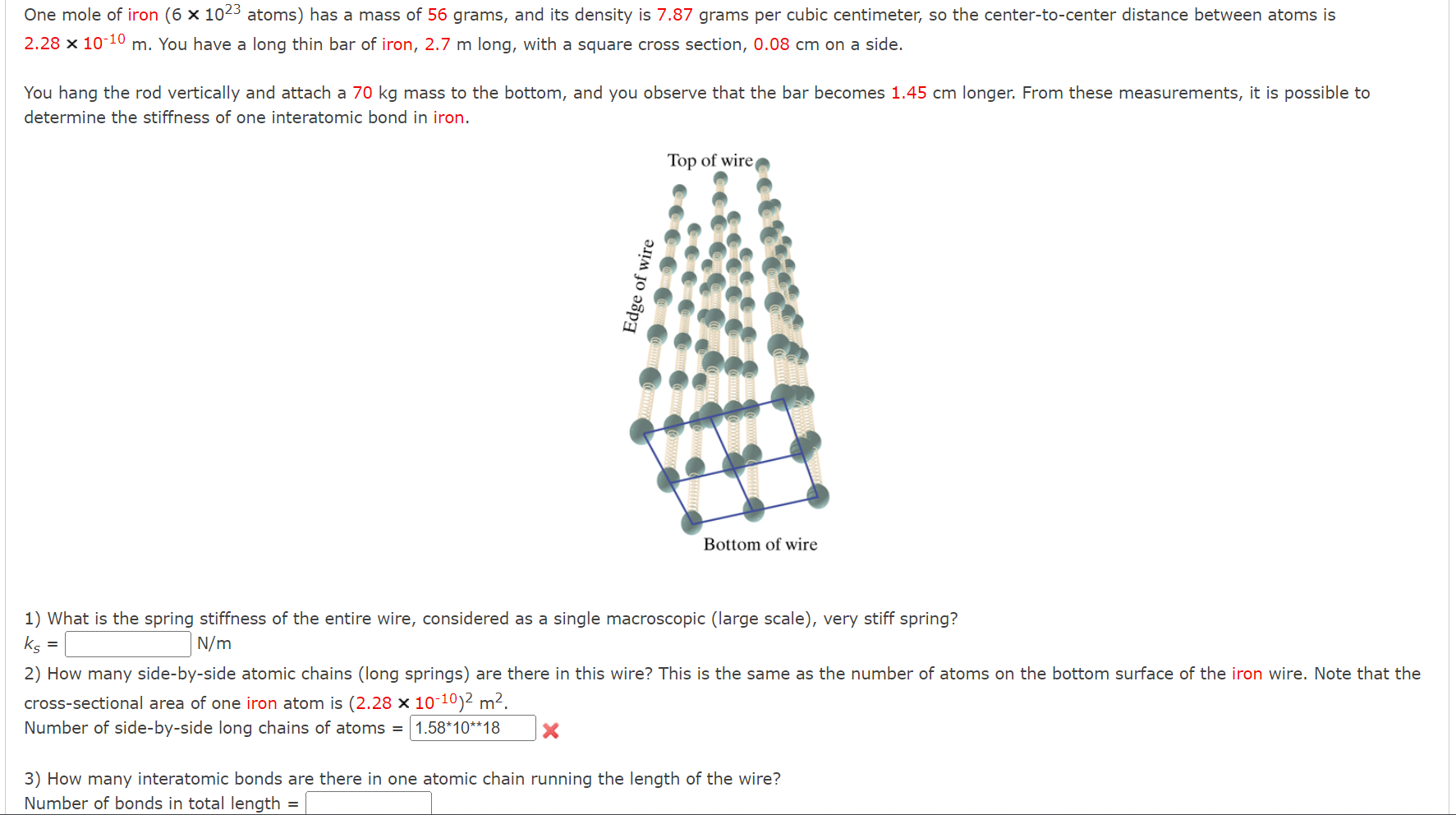
One mole of iron (6 1023 atoms) has a mass of 56 grams, and its density is 7.87 grams per cubic centimeter, so the center-to-center distance between atoms is 2.28 10-10 m. You have a long thin bar of iron, 2.7 m long, with a square cross section, 0.08 cm on a side. You hang the rod vertically and attach a 70 kg mass to the bottom, and you observe that the bar becomes 1.45 cm longer. From these measurements, it is possible to determine the stiffness of one interatomic bond in iron. Edge of wire Top of wire Bottom of wire 1) What is the spring stiffness of the entire wire, considered as a single macroscopic (large scale), very stiff spring? ks = N/m 2) How many side-by-side atomic chains (long springs) are there in this wire? This is the same as the number of atoms on the bottom surface of the iron wire. Note that the cross-sectional area of one iron atom is (2.28 10-10) m. Number of side-by-side long chains of atoms = 1.58*10**18 3) How many interatomic bonds are there in one atomic chain running the length of the wire? Number of bonds in total length =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


