Answered step by step
Verified Expert Solution
Question
1 Approved Answer
One of the major reasons for engine oil degradation is the oxidation of the motor oil. To retard the degradation process, most oils contain an
One of the major reasons for engine oil degradation is the oxidation of the motor oil. To retard the degradation process, most oils contain an antioxidant. Without an inhibitor to oxidation present, the suggested mechanism at low temperatures is
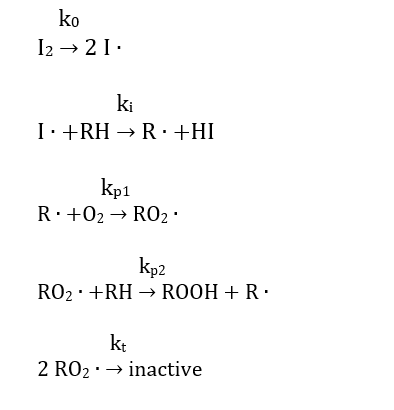
Where I2 is an initiator and RH is the hydrocarbon in the oil.
When an antioxidant is added to retard degradation at low temperatures, the following additional termination steps occur:
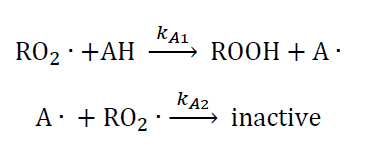
- Derive a rate law for the degradation of the motor oil in the absence of an antioxidant at low temperatures
- Derive a rate law for the rate of degradation of the motor oil in the presence of an antioxidant for low temperatures.
- How would your answer to part (a) change if the radicals I were produced at a constant rate in the engine and then found their way into the oil?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


