Answered step by step
Verified Expert Solution
Question
1 Approved Answer
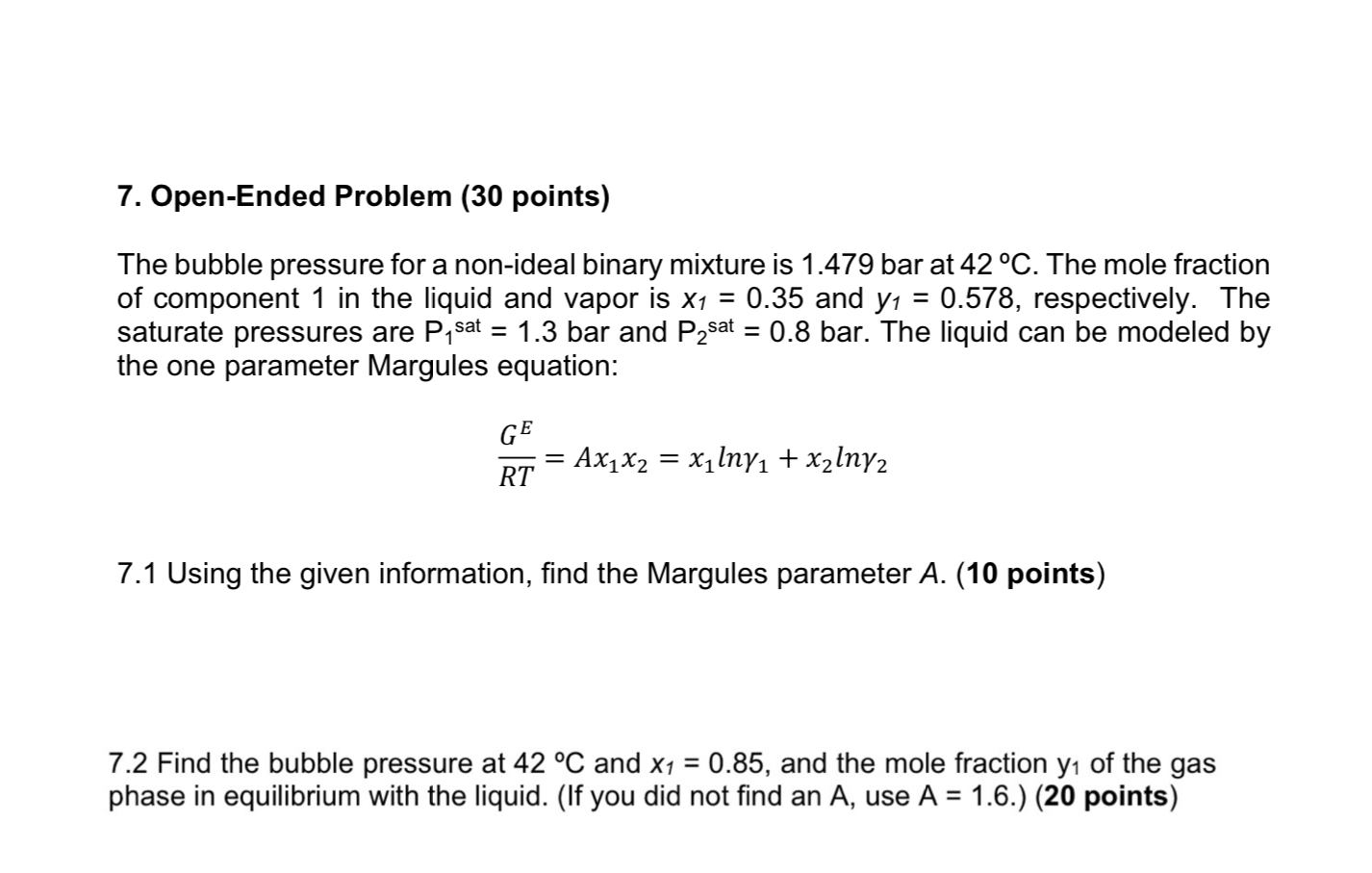
Open - Ended Problem ( 3 0 points ) The bubble pressure for a non - ideal binary mixture is 1 . 4 7 9
OpenEnded Problem points
The bubble pressure for a nonideal binary mixture is bar at The mole fraction of component in the liquid and vapor is and respectively. The saturate pressures are bar and bar. The liquid can be modeled by the one parameter Margules equation:
Using the given information, find the Margules parameter A points
Find the bubble pressure at and and the mole fraction of the gas phase in equilibrium with the liquid. If you did not find an use points
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


