Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Outdoor air at 30CDB and 85% RH and recirculation air at 22CDB and 63% RH are supplying into the mixing chamber as shown in
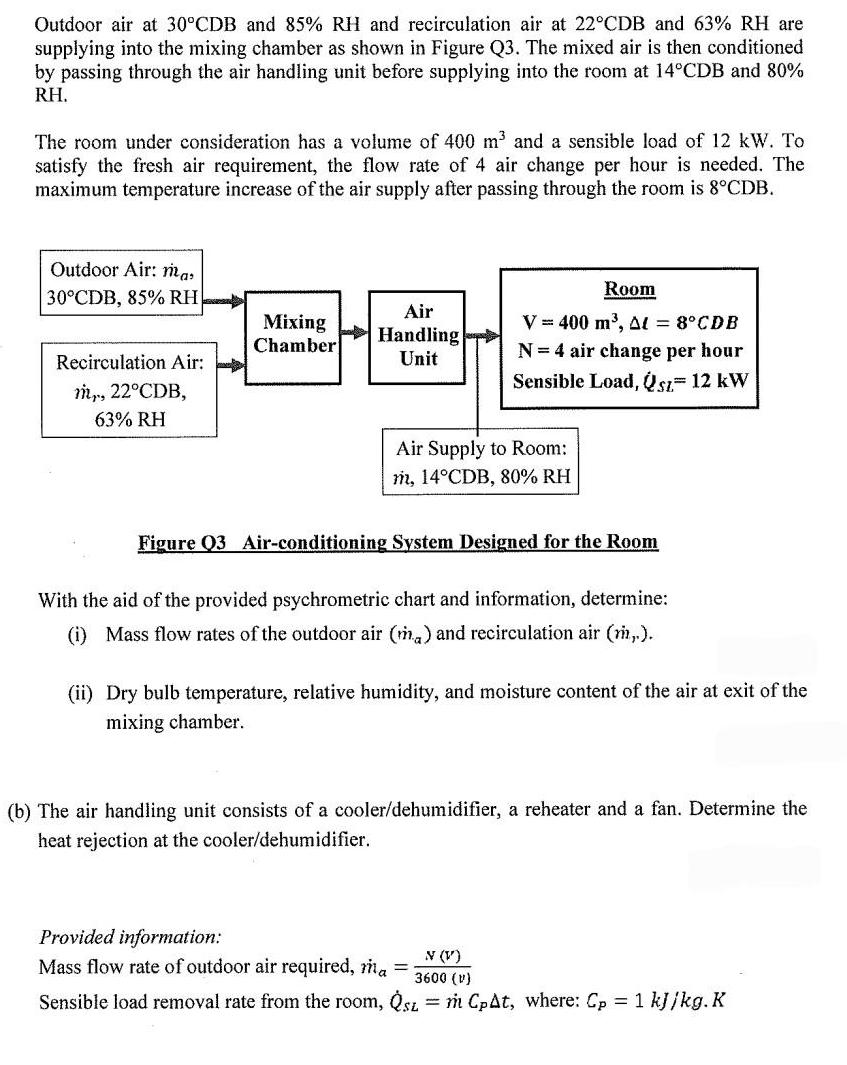
Outdoor air at 30CDB and 85% RH and recirculation air at 22CDB and 63% RH are supplying into the mixing chamber as shown in Figure Q3. The mixed air is then conditioned by passing through the air handling unit before supplying into the room at 14CDB and 80% RH. The room under consideration has a volume of 400 m and a sensible load of 12 kW. To satisfy the fresh air requirement, the flow rate of 4 air change per hour is needed. The maximum temperature increase of the air supply after passing through the room is 8CDB. Outdoor Air: mas 30CDB, 85% RH Recirculation Air: m,, 22CDB, 63% RH Mixing Chamber Air Handling Unit Room V = 400 m, A = 8CDB N = 4 air change per hour Sensible Load, Q sz= 12 kW Air Supply to Room: m, 14CDB, 80% RH Figure 03 Air-conditioning System Designed for the Room With the aid of the provided psychrometric chart and information, determine: (1) Mass flow rates of the outdoor air (m) and recirculation air (m,.). (ii) Dry bulb temperature, relative humidity, and moisture content of the air at exit of the mixing chamber. (b) The air handling unit consists of a cooler/dehumidifier, a reheater and a fan. Determine the heat rejection at the cooler/dehumidifier. Provided information: Mass flow rate of outdoor air required, ma = N (V) 3600 (V) Sensible load removal rate from the room, sz = m CpAt, where: Cp = 1 kJ/kg.K
Step by Step Solution
★★★★★
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
To solve the given problem well use the provided information and psychrometric chart Heres the stepb...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


