Answered step by step
Verified Expert Solution
Question
1 Approved Answer
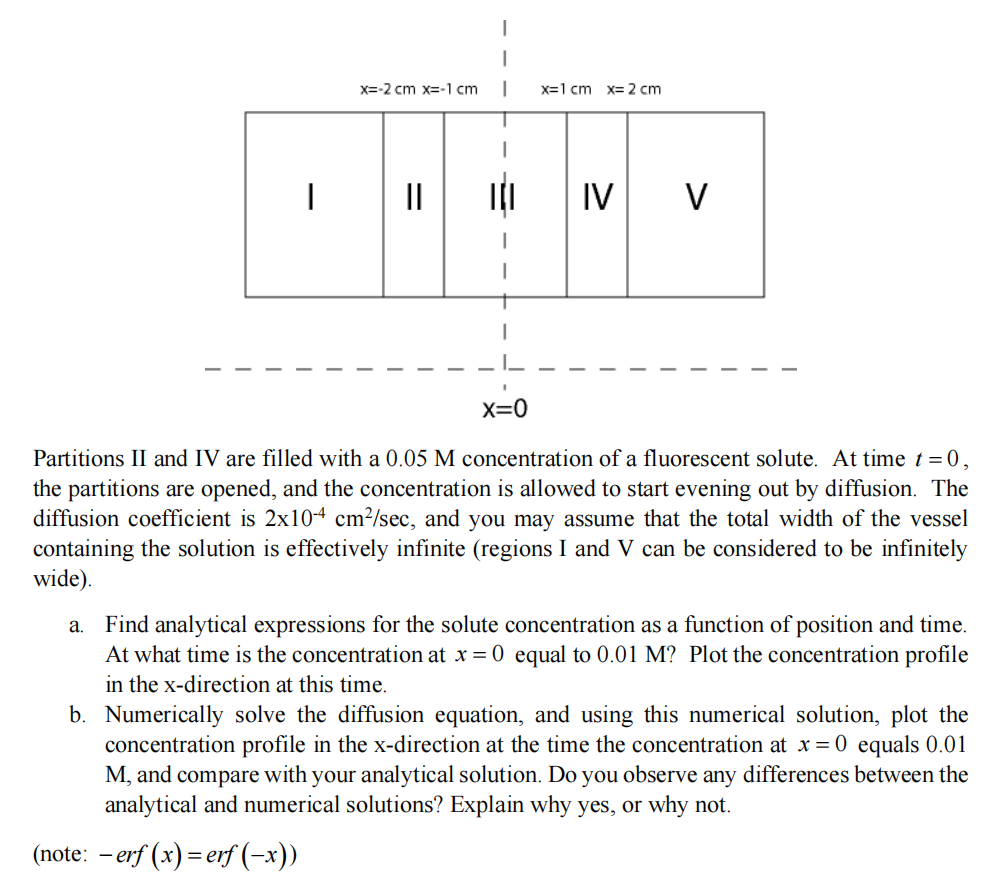
Partitions II and IV are filled with a 0 . 0 5 M concentration of a fluorescent solute. At time t = 0 , the
Partitions II and IV are filled with a concentration of a fluorescent solute. At time the partitions are opened, and the concentration is allowed to start evening out by diffusion. The diffusion coefficient is and you may assume that the total width of the vessel containing the solution is effectively infinite regions I and V can be considered to be infinitely wide
a Find analytical expressions for the solute concentration as a function of position and time. At what time is the concentration at equal to Plot the concentration profile in the direction at this time.
b Numerically solve the diffusion equation, and using this numerical solution, plot the concentration profile in the direction at the time the concentration at equals and compare with your analytical solution. Do you observe any differences between the analytical and numerical solutions? Explain why yes, or why not.
note: erferf
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


