Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer A chemist wants to perform a reaction using the reagent below, which can act as a Bransted-Lowry base. Considering only the reagent's capacity
please answer 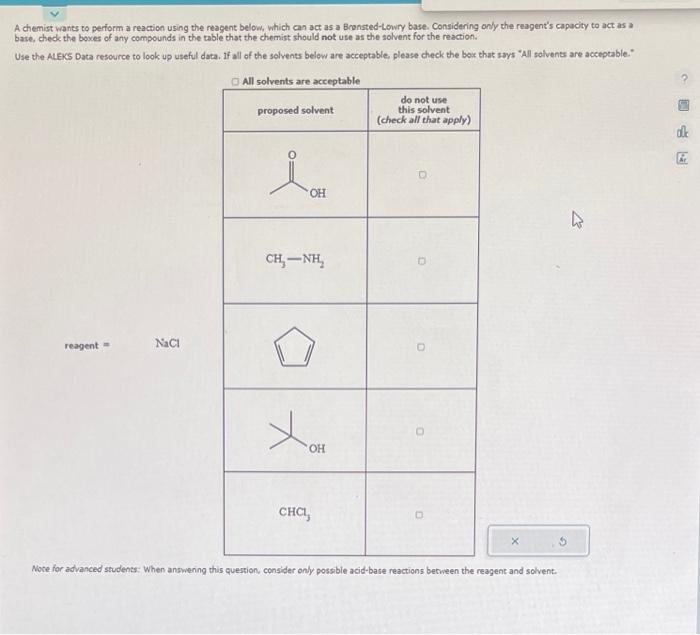
A chemist wants to perform a reaction using the reagent below, which can act as a Bransted-Lowry base. Considering only the reagent's capacity to act as a base, chede the boxes of any compounds in the table that the chemist should not use as the solvent for the reaction. Use the ALEks Data resource to look up useful data. If all of the solvents below are acceptable. please check the bock that says "All solvents are acceptable 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


