Question: please answer E and F. I have posted my calculated values for the information needed (although it may be modified if i put in wrong

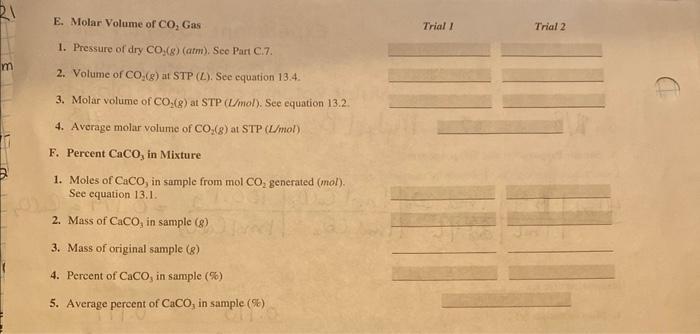

A. Sample Preparation and Setup Apparatus Calculation of mass of CaCO3 sample for analysis (from Prelaboratory Assignment, question 2). 3. Instructor's approval of apparatus C. Determination of Volume, Temperature, and Pressure of the Carbon Dioxide Gas F. Percent CaCO3 in Mixture Molar Volume of Carbon The mass loss of the CaCO3 mixture is due to the mass of CO2(g) evolved in the reacDioxide tion; the mass of CO2 is converied to moles of CO2 evolved. The volume of CO2(g), collected over water under the temperature and pressure conditions of the experiment, is calculated at STP conditions (see equation 13.4). Knowing the number of moles and the volume at STP, the molar volume of CO2(g) is calculated. nCO3VCO1(STP)=molarvolumeofCO2 Volume of Collected The CO2(g) evolved in the reaction is collected by displacing an equal volume of water Carbon Dioxide at STP [Figure 13.2 (top)]. Because the CO2(g) is bubbled through the water, it is considered Dalion's law of porfial pressures: The "wel," meaning that the volume oecupied by the CO2(g) is also saturated with water ratal pressure, Pt exered by a vapor at the temperature of the water over which it is collected. Therefore, the total midure of gasess is the sum of the pressure, PT, in this volume is due to the combined pressures of the CO2 gas, PCo, and individual pressures falled partial the water vapor, pugo. The pressure of the dry CO2 is calculated using Dalton's law of pressures) exerted by eoch of the partial pressures. constitivent goses For carbon dioxide gas, Pco,eser=PTpHo The pressure of the water vapor, Pito, at the gas-colleeting temperature (obtained from Appendix C) is subtracted from the total pressure of the gases, PT, in the gascollecting vessel. Experimentally, the total pressure of the gaseous mixture (CO2+ H2O) is adjusted to atmospheric pressure (PY=Pmam) by adjusting the water levels inside and outside the gas-collecting vessel to be equal [Figure 13.2 (bottom)]. Atmospheric pressure is read from the laboratory barometer. Once the experimental values for the volume, pressure, and temperature of the CO2(g) are determined, the volume of CO2(g) at STP conditions is calculated using a combination of Boyle's law (P1/V) and Charles' law (VT). VCO2(atSTP)=VCO2E(760tortpCo1app(forr))(TCO2ap(K)273K)BoyleslawCharleslawcorrectioncorrection This value is used in equation 13.2. In addition to the fact that the collected CO2(g) is wet, CO2 also has an appreciable solubility in water. When the CO2(g) is bubbled through pure water, some of the CO2 dissolves in the water and is not measured as gas evolved in the reaction. To minimize Figure 13.2 The collection of any loss of evolved CO2 as a result of its water solubility, the water is saturated with CO2 gas over water (fop) and the CO2 before the experiment is conducted. This saturation is completed with the addition pressure meosurement of "wet" of either sodium bicarbonate to slightly acidified water or an antacid tablet that evolves CO2 gas (bottom). CO2, such as Alka-Seltzer. Percent Calcium Carbonate By use of equation 13.1, once the moles of CO2(g) evolved in the reaction is known, in Mixture the moles and mass of CaCO3 in the heterogeneous mixture can be calculated. The percent CaCO3 in the mixture is calculated by dividing this mass of CaCO3 by that of the original mixture and multiplying by 100 : massofmixturemassofCaCO7100=%CaCO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


