Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please complete and submit the History of the Periodic Table Worksheet. Download History of the Periodic Table Worksheet. The PowerPoint in Unit 2 Resources can
Please complete and submit the History of the Periodic Table Worksheet. Download History of the Periodic Table Worksheet. The PowerPoint in Unit 2 Resources can help you complete this task.
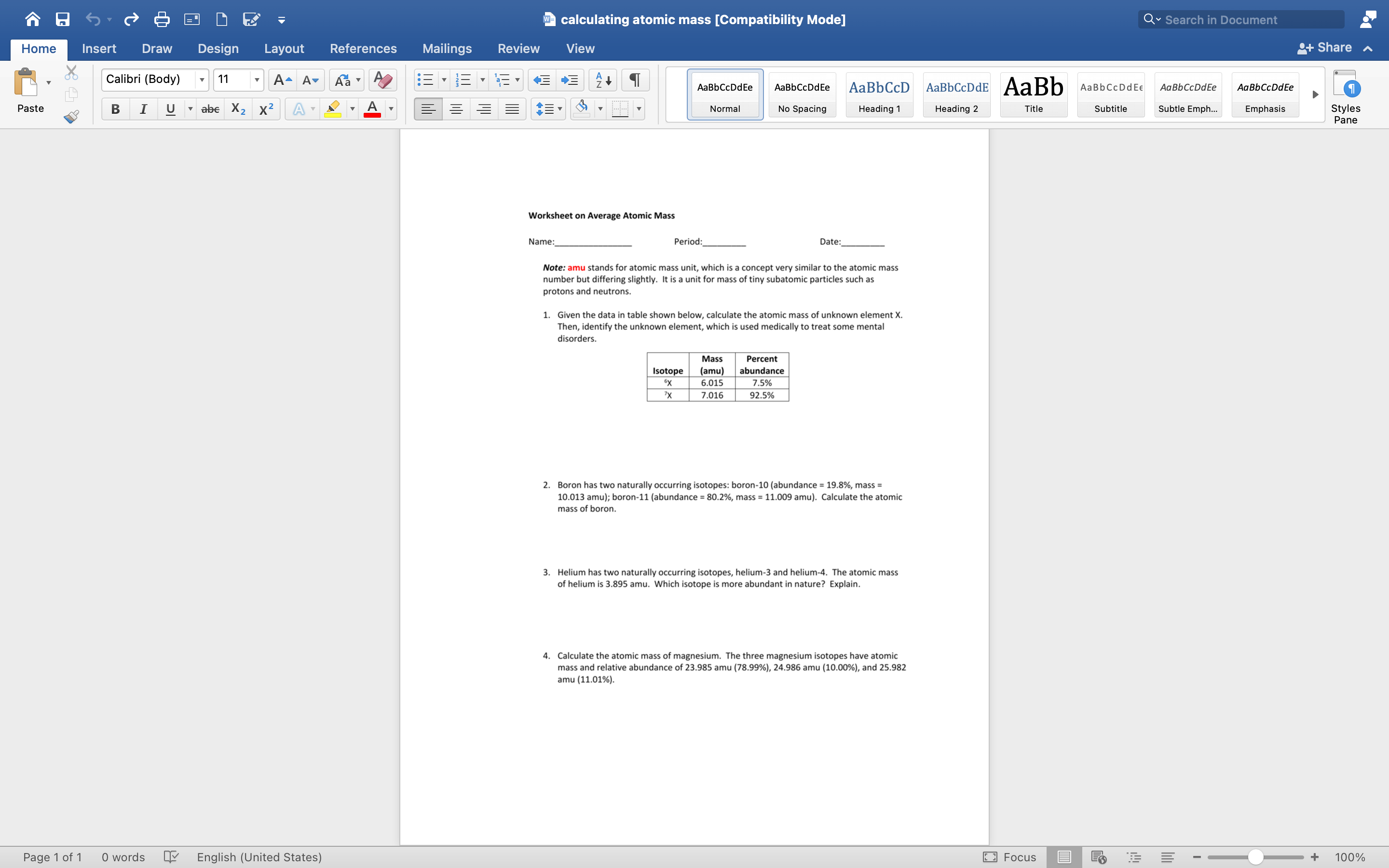 Note: amu stands for atomic mass unit, which is a concept very similar to the atomic mass number but differing slightly. It is a unit for mass of tiny subatomic particles such as protons and neutrons. 1. Given the data in table shown below, calculate the atomic mass of unknown element X. Then, identify the unknown element, which is used medically to treat some mental disorders. 2. Boron has two naturally occurring isotopes: boron- 10 (abundance =19.8%, mass = 10.013amu ); boron-11 (abundance =80.2%, mass =11.009amu ). Calculate the atomic mass of boron. 3. Helium has two naturally occurring isotopes, helium-3 and helium-4. The atomic mass of helium is 3.895 amu. Which isotope is more abundant in nature? Explain. 4. Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic mass and relative abundance of 23.985amu(78.99%),24.986amu(10.00%), and 25.982 amu (11.01\%)
Note: amu stands for atomic mass unit, which is a concept very similar to the atomic mass number but differing slightly. It is a unit for mass of tiny subatomic particles such as protons and neutrons. 1. Given the data in table shown below, calculate the atomic mass of unknown element X. Then, identify the unknown element, which is used medically to treat some mental disorders. 2. Boron has two naturally occurring isotopes: boron- 10 (abundance =19.8%, mass = 10.013amu ); boron-11 (abundance =80.2%, mass =11.009amu ). Calculate the atomic mass of boron. 3. Helium has two naturally occurring isotopes, helium-3 and helium-4. The atomic mass of helium is 3.895 amu. Which isotope is more abundant in nature? Explain. 4. Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic mass and relative abundance of 23.985amu(78.99%),24.986amu(10.00%), and 25.982 amu (11.01\%) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


