Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me asweing this step by step, thanks in advance!! :) -Answers for a) and b) are displayed in the 2nd picture. For the
Please help me asweing this step by step, thanks in advance!! :) 
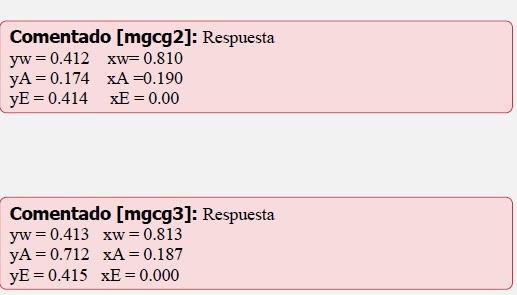
For the reaction: C2H4+H2OC2H5OH at 254C, y 100atm, calculate the composition of the liquid and vapor phases which are in equilibrium under these conditions. Assume the reactants initially consisted of a stoichiometric mixture of C2H4 y H2O. a. Calculate from K=0.00743 for G referred to the following standard states: C2H5OH, unit fugacity, 254C C2H4, unit fugacity, 254C H2O, unit fugacity, 254C b. Calculate from K=0.00600 for G referred to the following standard states: C2H5OH, liquid, pure, 254C C2H4, unit fugacity, 254C H2O, liquid, pure, 254C Ideal solutions, but not ideal gases may be assumed. Additional data follow: Vapor Pressures at 254C C2H5OH:76atm H2O:42atm C2H4 : infinite (f/p) ratios at 254C C2H5OH at vapor pressure: 0.59 at total pressure: 0.49 H2O at vapor pressure: 0.86 at total pressure: 0.71 C2H4 at total pressure: 0.94 Comentado[mgcg2]:Respuestayw=0.412xw=0.810yA=0.174xA=0.190yE=0.414xE=0.00Comentado[mgcg3]:Respuestayw=0.413xw=0.813yA=0.712xA=0.187yE=0.415xE=0.000 -Answers for a) and b) are displayed in the 2nd picture.


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


