Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please I need one sample calculation for table 3 and 4 I just want table 3 and 4 please Rims with 3 drops of (H)MO0catalyst
please I need one sample calculation for table 3 and 4 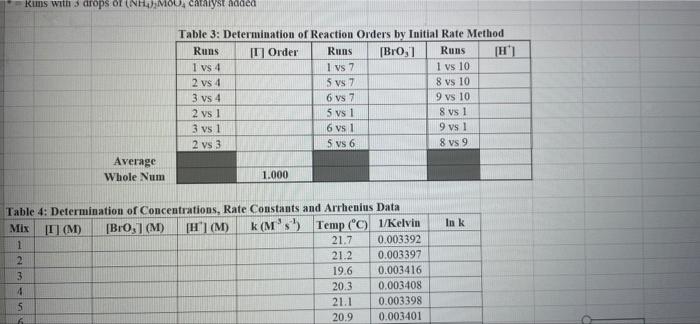



I just want table 3 and 4 please 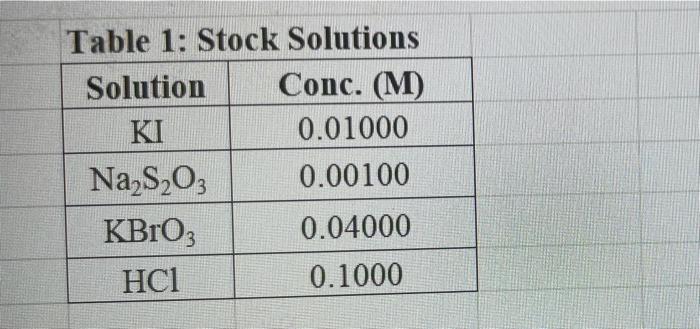
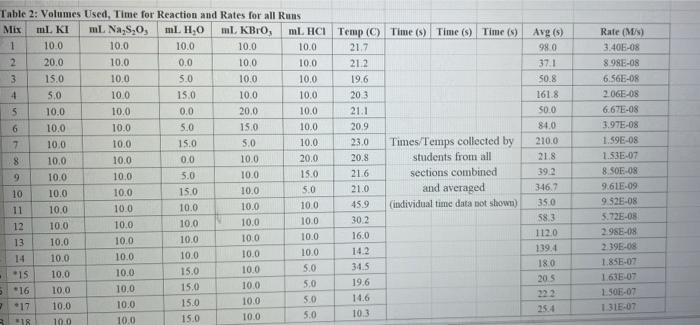
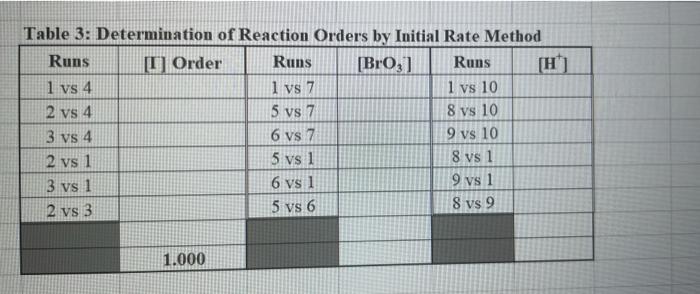
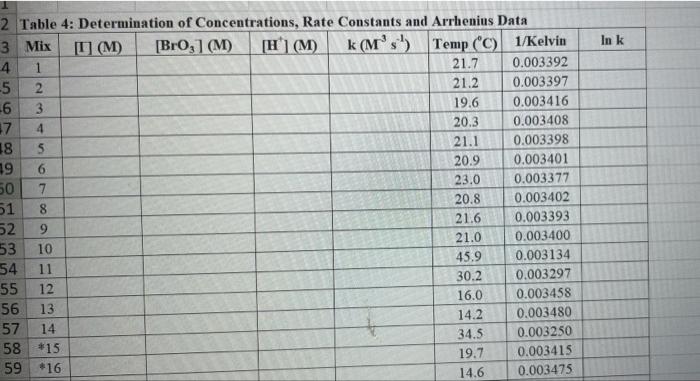
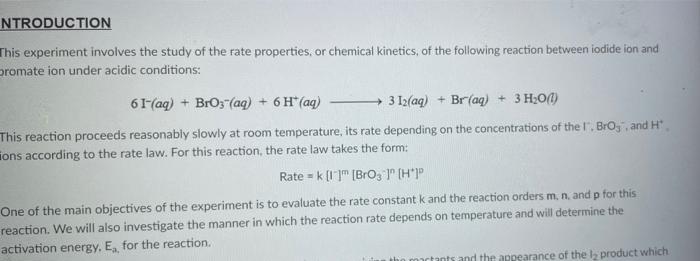
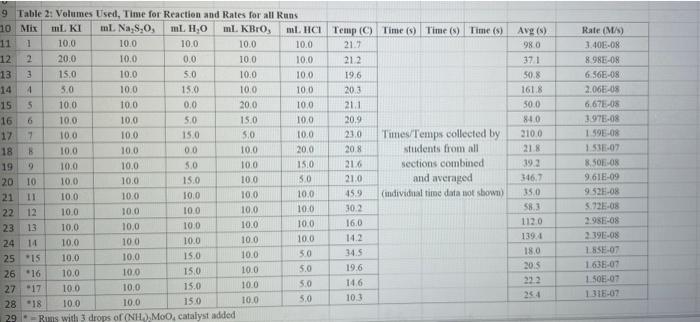
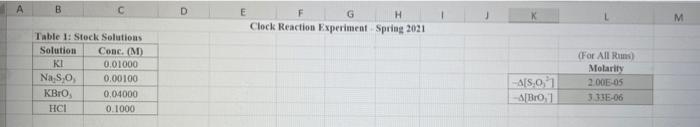
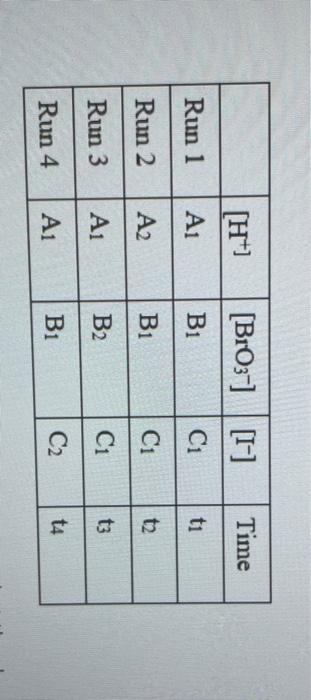
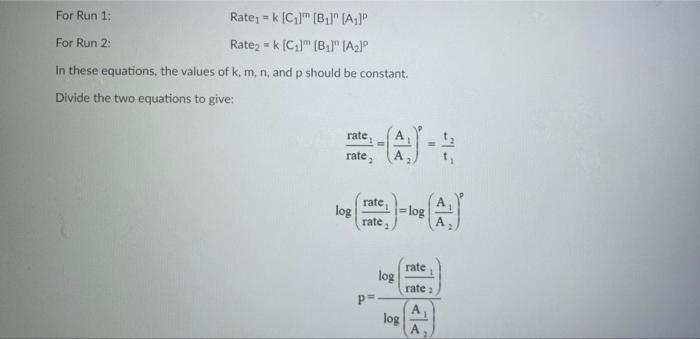
Rims with 3 drops of (H)MO0catalyst added Table 3: Determination of Reaction Orders by Initial Rate Method Runs IT] Order Runs [Bro, Runs I vs 4 I vs 7 1 1 vs 10 2 vs 4 5 vs 7 8 vs 10 3 vs 4 6 vs 7 9 vs 10 2 vs 1 5 vs 1 8 vs 1 3 vs 1 6 vs 1 9 vs 1 2 vs 3 S vs 6 8 vs 9 Average Whole Num 1.000 In k Table 4: Determination of Concentrations, Rate Constants and Arrhenius Data Mix [T](M) [Bro, (M) [H(M) k( Ms) Temp (C) 1/Kelvin 1 21.7 0.003392 2 21.2 0.003397 3 19.6 0.003416 4 20.3 0.003408 5 21.1 0.003398 6 20.9 0.003401 9 Table 2: Volumes Used, Time for Reaction and Rates for all Runs 10 Mix mL KI ml. Na,s,o, mL HO ml. KBrO; ml HCI Temp (C) Time (s) Time () Time (5) 11 1 100 10.0 10.0 10.0 10.0 21.7 12 2 200 10.0 0.0 10.0 10.0 212 13 3 15.0 10.0 5.0 10.0 10.0 19.6 14 4 3.0 10.0 15.0 10.0 100 20.3 15 5 10.0 10.0 0.0 20.0 100 21.1 16 6 10.0 10.0 5.0 15.0 10.0 20.9 17 7 10.0 10.0 15.0 5.0 10.0 23.0 Times/Temps collected by 18 B 100 10.0 0.0 10.0 20.0 20.8 students from all 19 10.0 10.0 5.0 10.0 15.0 21.6 sections combined 20 10 10.0 10.0 15.0 100 50 21.0 and averaged 21 II 10.0 10.0 100 10.0 10.0 45.9 (individual time data not shown) 22 12 100 10.0 10.0 10.0 10.0 302 23 13 10.0 10.0 10.0 10.0 10.0 16.0 24 14 10.0 100 10.0 10.0 100 14.2 25 '15 10.0 10.0 15.0 10.0 50 34.5 26 '16 10.0 10.0 15.0 10.0 5.0 19.6 27 "17 10.0 10,0 15.0 10.0 5.0 146 28 '18 10.0 10.0 150 100 5.0 10.3 29 - Runs with 3 drops of (NH). Mo, catalyst added Avg (8) 98.0 371 50.8 161.8 50.0 8-40 210.0 218 39.2 346.7 35.0 58.3 112.0 1394 18.0 20.5 232 254 Rate (MS) 3.40E-08 8.98E-08 6.56E-08 2.065-08 6.67E-05 1.97E-08 1.59E-08 1.536-07 8.50-08 9.61-09 9525-08 5.72E-08 2.985-08 2.39E-08 1.85-07 1638-07 1.505-07 1 31-07 B D 1 E G H Clock Reaction Experiment Spring 2021 M Table 1: Stock Solutions Solution Conc. (M) KI 0.01000 Na S, 0.00100 KBro, 0.04000 HCI 0.1000 -Also A BO 1 (For All Runs Molarity 2.00E-05 3336-06 [B103] [1-] Time [H] A1 Run 1 B1 Ci ti Run 2 A2 B1 C1 t2 Run 3 A1 B2 C1 t3 Run 4 A1 Bi C2 t4 For Run 1: Rate = k (CMB [A]P For Run 2: Rate = k (C)(Bl" (A2) In these equations, the values of k, m, n, and p should be constant. Divide the two equations to give: rate A rate, rate log log rate rate log rate : p= A log Table 1: Stock Solutions Solution Conc. (M) KI 0.01000 Na2S203 0.00100 KBrO3 0.04000 HC1 0.1000 Table 2: Volumes Used, Time for Reaction and Rates for all Runs Mix ml, KI ml Na,s,o, ml H.O ml, KBro, ml. HC Temp (0) Time () Time (9) Time (s) 1 100 10.0 10.0 10.0 10.0 21.7 2 20.0 10.0 0.0 10.0 10.0 212 3 15.0 10.0 5.0 10.0 10,0 19.6 4 5.0 10.0 15.0 10.0 10.0 203 5 10.0 10.0 0.0 20.0 10.0 21.1 6 10.0 10.0 5.0 15.0 10.0 20.9 7 10.0 10.0 15.0 5.0 10.0 23.0 Times/Temps collected by 8 10.0 10:0 0.0 10.0 20.0 20.8 students from all 9 10.0 5.0 10.0 15.0 21.6 sections combined 10 10.0 10.0 15.0 10.0 5.0 21.0 and averaged 11 10.0 10.0 10.0 10.0 10.0 45.9 (individual time data not shown) 12 10.0 10.0 10.0 10.0 10.0 30.2 13 10.0 10.0 10.0 100 10.0 16.0 14 10.0 10.0 10.0 10.0 14.2 15 10.0 10.0 15.0 34.5 10.0 5.0 10.0 5 16 10.0 15.0 5,0 10.0 19.6 2.17 10.0 10.0 15.0 10.0 5.0 10.0 10.0 15.0 10.0 5.0 10.3 10.0 Avg (5) 980 37.1 50.8 161.8 50.0 84.0 210.0 21.8 39.2 346.7 35.0 583 112.0 139.4 180 20.5 222 25.4 Rate (M) 3.405-08 8.98E-08 6.566-08 2.06E-08 6.67E-08 3.97E-08 1.59E-08 1.53E-07 8.SOE-08 9.61E-09 9.525-08 5.72E-08 2.98E-ON 2.39E-08 1.856-07 1.635-07 1505-07 131E-07 10.0 14,6 5 18 Table 3: Determination of Reaction Orders by Initial Rate Method Runs [T] Order Runs [Br03] Runs 1 vs 4 1 vs 7 1 vs 10 2 vs 4 5 vs 7 8 vs 10 3 vs 4 6 vs 7 9 vs 10 2 vs 1 5 vs 1 8 vs 1 3 vs 1 6 vs 1 9 vs 1 2 vs 3 5 vs 6 8 vs 9 1.000 In k co oo 2 Table 4: Determination of Concentrations, Rate Constants and Arrhenius Data 3 Mix [T](M) [BrO(M) [H](M) k(M's) Temp (C) 1/Kelvin 4 1 21.7 0.003392 -5 2 21.2 0.003397 -6 3 19.6 0.003416 7 4 20.3 0.003408 8 5 21.1 0.003398 19 6 20.9 0.003401 50 7 23.0 0.003377 51 8 20.8 0.003402 52 9 21.6 0.003393 53 10 21.0 0.003400 54 11 45.9 0.003134 55 30.2 0.003297 12 0.003458 56 16.0 13 14.2 0.003480 57 14 34.5 0.003250 58 *15 19.7 0.003415 59 16 14.6 0.003475 NTRODUCTION This experiment involves the study of the rate properties, or chemical kinetics of the following reaction between iodide ion and romate ion under acidic conditions: 61- (aq) + BrO3(aq) + 6 H+ (aq) 3 12(aq) + Br(aq) + 3 H20(1) This reaction proceeds reasonably slowly at room temperature, its rate depending on the concentrations of the I. Broj and H Fons according to the rate law. For this reaction, the rate law takes the form: Rate = k [11" (Broz 1" (H*P One of the main objectives of the experiment is to evaluate the rate constant k and the reaction orders m, n, and p for this reaction. We will also investigate the manner in which the reaction rate depends on temperature and will determine the activation energy. E, for the reaction tants and the appearance of the la product which 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


