Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve ASAP A body-centered cubic unit cell has a volume of 3.931023cm3. Find the radius of the atom in pm. Express your answer in
please solve ASAP 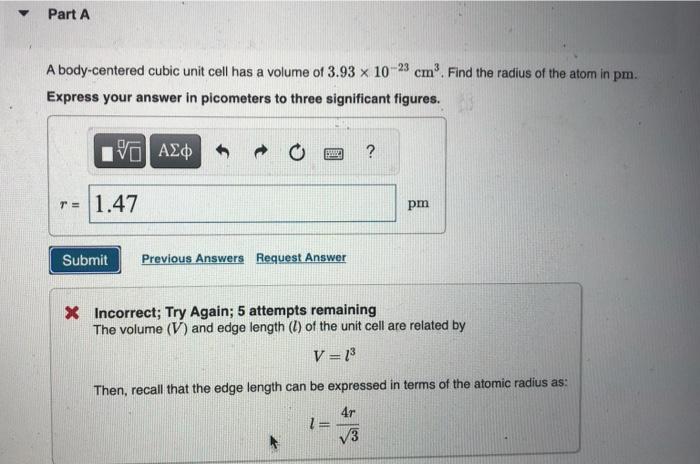
A body-centered cubic unit cell has a volume of 3.931023cm3. Find the radius of the atom in pm. Express your answer in picometers to three significant figures. 23 Incorrect; Try Again; 5 attempts remaining The volume (V) and edge length (l) of the unit cell are related by V=l3 Then, recall that the edge length can be expressed in terms of the atomic radius as: l=34r 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


