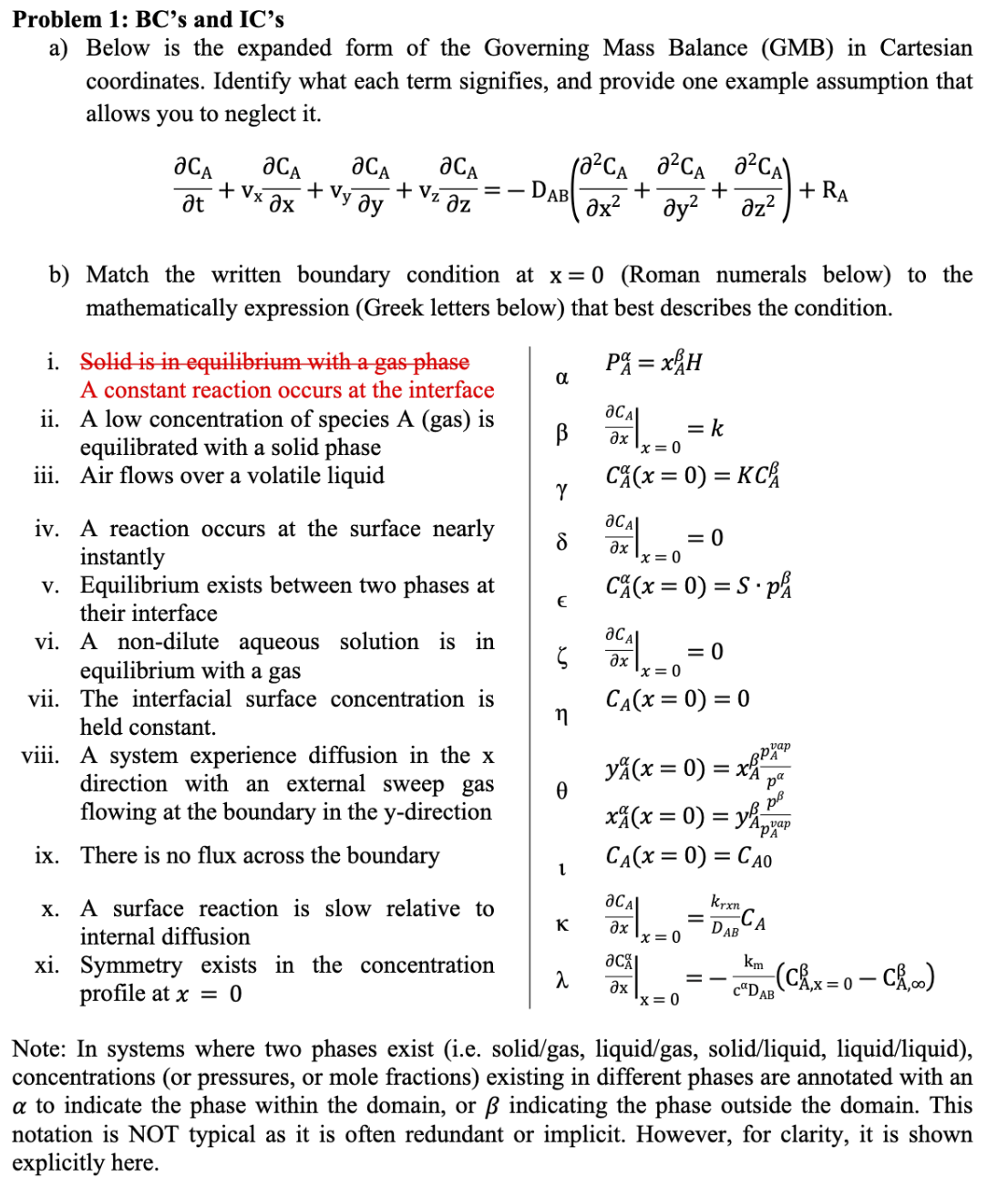
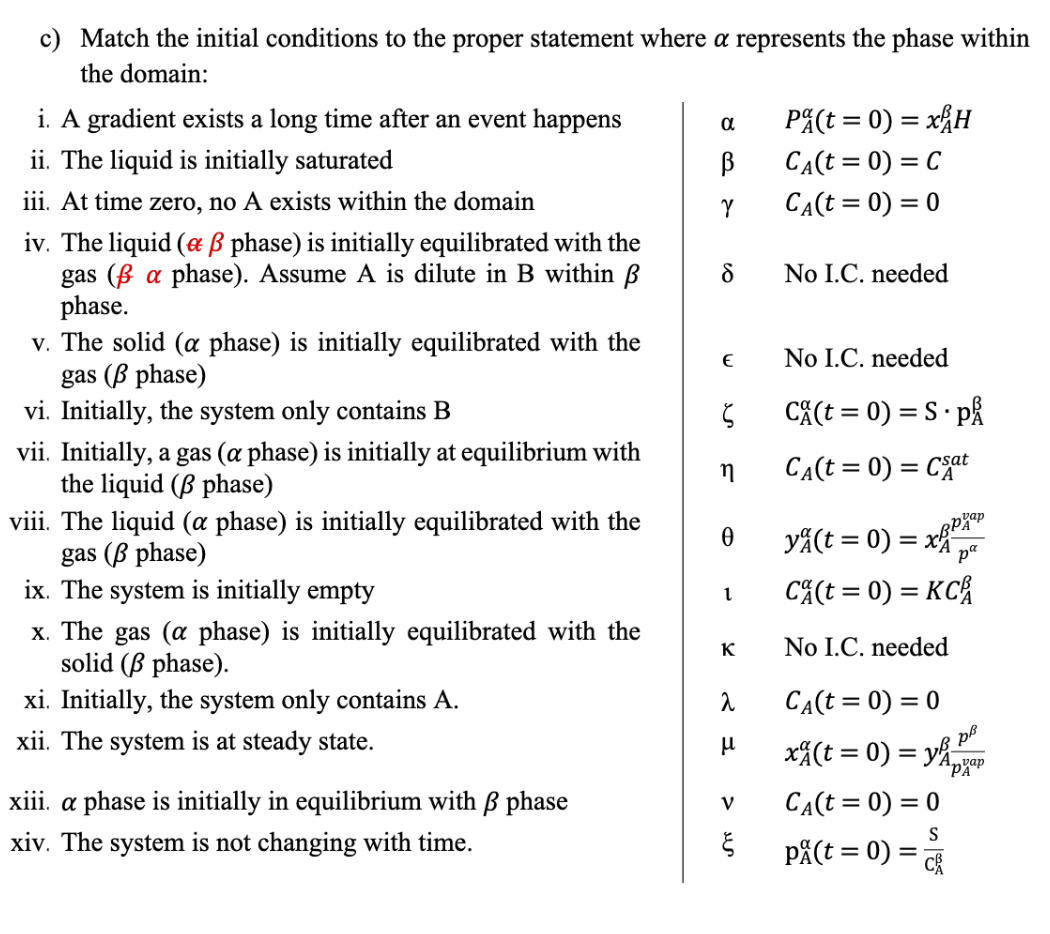
Problem 1: BC's and IC's a) Below is the expanded form of the Governing Mass Balance (GMB) in Cartesian coordinates. Identify what each term signifies, and provide one example assumption that allows you to neglect it. tCA+vxxCA+vyyCA+vzzCA=DAB(x22CA+y22CA+z22CA)+RA b) Match the written boundary condition at x=0 (Roman numerals below) to the mathematically expression (Greek letters below) that best describes the condition. i. Solid is in equilibrium with a gas phase PA=xAH A constant reaction occurs at the interface ii. A low concentration of species A (gas) is equilibrated with a solid phase xCAx=0=k iii. Air flows over a volatile liquid CA(x=0)=KCA iv. A reaction occurs at the surface nearly instantly v. Equilibrium exists between two phases at their interface vi. A non-dilute aqueous solution is in equilibrium with a gas vii. The interfacial surface concentration is CA(x=0)=0 held constant. viii. A system experience diffusion in the x direction with an external sweep gas flowing at the boundary in the y-direction ix. There is no flux across the boundary x. A surface reaction is slow relative to internal diffusion xi. Symmetry exists in the concentration profile at x=0 yA(x=0)=xAppApapxA(x=0)=yApAappCA(x=0)=CA0xCAx=0=DABkxxnCAxCAx=0=cDABkm(CA,x=0CA,) Note: In systems where two phases exist (i.e. solid/gas, liquid/gas, solid/liquid, liquid/liquid), concentrations (or pressures, or mole fractions) existing in different phases are annotated with an to indicate the phase within the domain, or indicating the phase outside the domain. This notation is NOT typical as it is often redundant or implicit. However, for clarity, it is shown explicitly here. c) Match the initial conditions to the proper statement where represents the phase within the domain: i. A gradient exists a long time after an event happens ii. The liquid is initially saturated iii. At time zero, no A exists within the domain iv. The liquid ( phase) is initially equilibrated with the gas ( phase). Assume A is dilute in B within No I.C. needed phase. v. The solid ( phase) is initially equilibrated with the No I.C. needed gas ( phase) vi. Initially, the system only contains B CA(t=0)=SpA vii. Initially, a gas ( phase) is initially at equilibrium with the liquid ( phase) CA(t=0)=CAsat viii. The liquid ( phase) is initially equilibrated with the gas ( phase) yA(t=0)=xAppAvap ix. The system is initially empty CA(t=0)=KCA x. The gas ( phase) is initially equilibrated with the No I.C. needed solid ( phase). xi. Initially, the system only contains A. CA(t=0)=0 xii. The system is at steady state. xA(t=0)=yApAvapp xiii. phase is initially in equilibrium with phase v CA(t=0)=0 xiv. The system is not changing with time. pA(t=0)=CAS








