Answered step by step
Verified Expert Solution
Question
1 Approved Answer
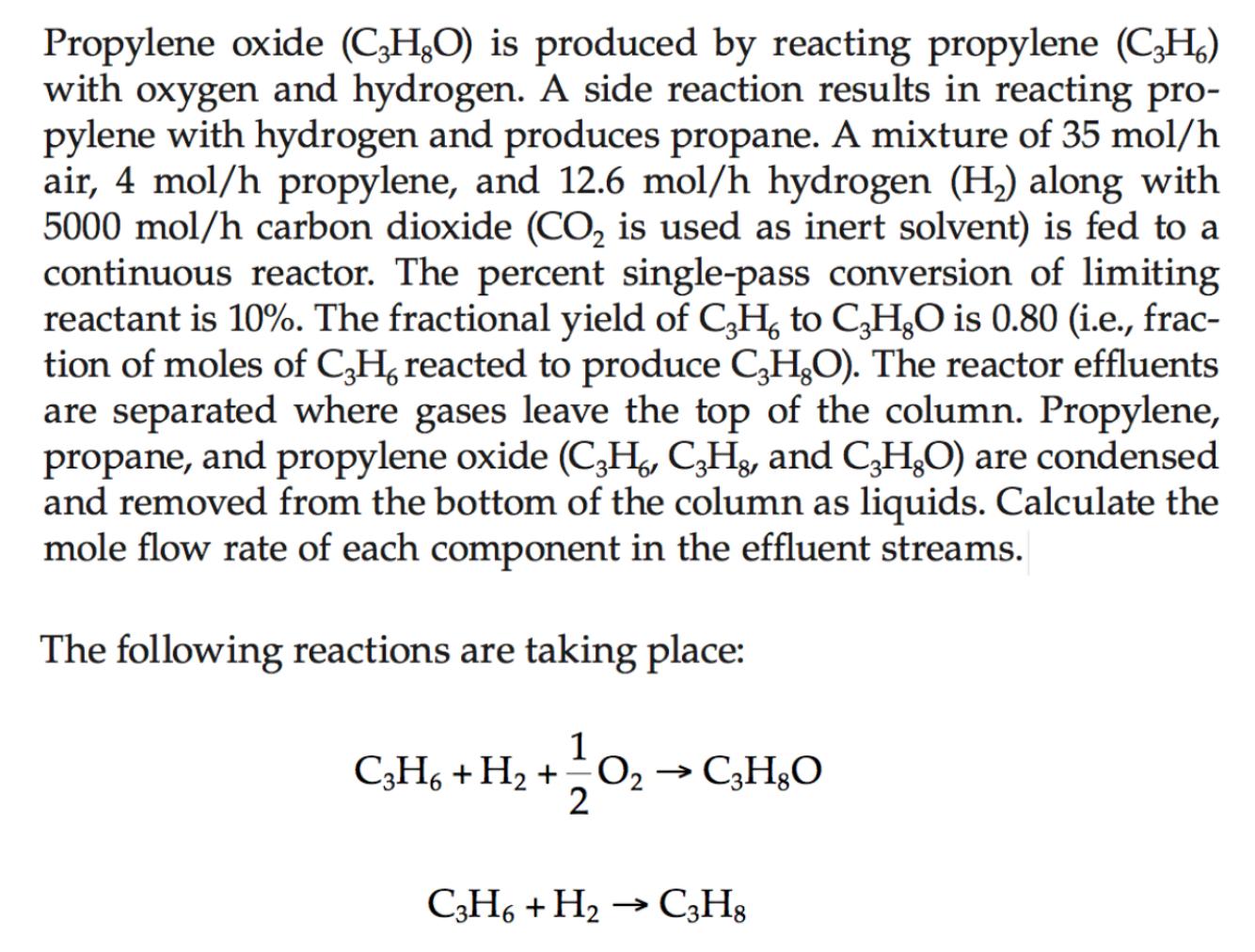
Propylene oxide ( C 3 H 8 O ) is produced by reacting propylene ( C 3 H 6 ) with oxygen and hydrogen. A
Propylene oxide is produced by reacting propylene with oxygen and hydrogen. A side reaction results in reacting propylene with hydrogen and produces propane. A mixture of air, propylene, and hydrogen along with carbon dioxide is used as inert solvent is fed to a continuous reactor. The percent singlepass conversion of limiting reactant is The fractional yield of to is ie fraction of moles of reacted to produce The reactor effluents are separated where gases leave the top of the column. Propylene, propane, and propylene oxide and : are condensed and removed from the bottom of the column as liquids. Calculate the mole flow rate of each component in the effluent streams.
The following reactions are taking place:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


