Answered step by step
Verified Expert Solution
Question
1 Approved Answer
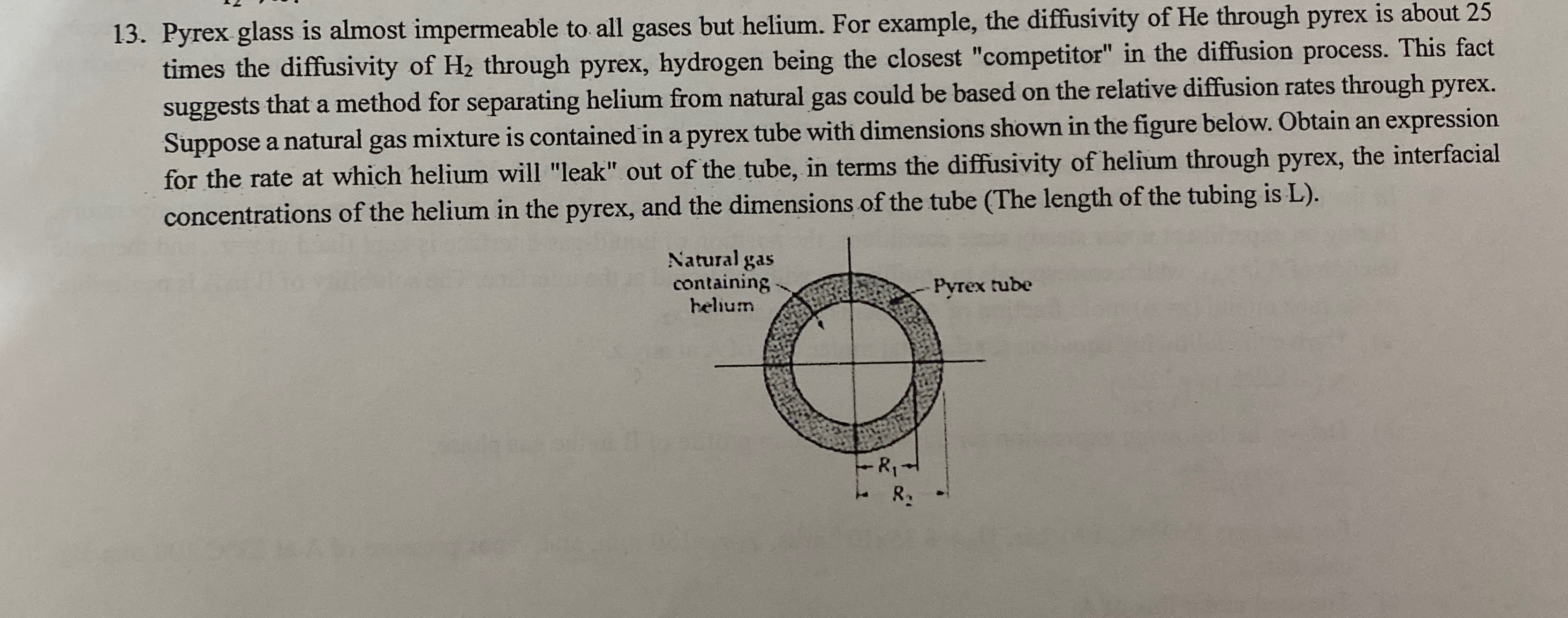
Pyrex glass is almost impermeable to all gases but helium. For example, the diffusivity of He through pyrex is about 2 5 times the diffusivity
Pyrex glass is almost impermeable to all gases but helium. For example, the diffusivity of He through pyrex is about times the diffusivity of through pyrex, hydrogen being the closest "competitor" in the diffusion process. This fact suggests that a method for separating helium from natural gas could be based on the relative diffusion rates through pyrex. Suppose a natural gas mixture is contained in a pyrex tube with dimensions shown in the figure below. Obtain an expression for the rate at which helium will "leak" out of the tube, in terms the diffusivity of helium through pyrex, the interfacial concentrations of the helium in the pyrex, and the dimensions of the tube The length of the tubing is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


