Answered step by step
Verified Expert Solution
Question
1 Approved Answer
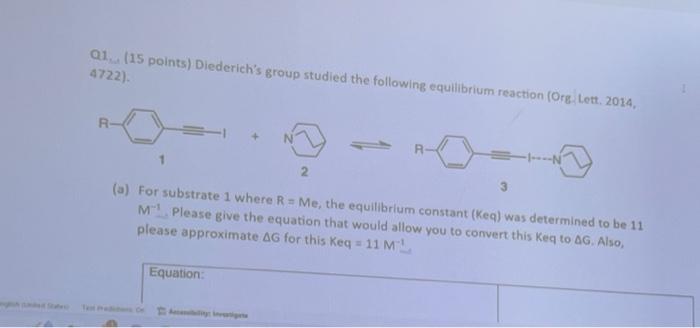
Q1. (15 points) Diederich's group studied the following equilibrium reaction (Org. Lett. 2014, 4722). 1 2 3 (a) For substrate 1 where ( mathrm{R}=mathrm{Me} ),
Q1. (15 points) Diederich's group studied the following equilibrium reaction (Org. Lett. 2014, 4722). 1 2 3 (a) For substrate 1 where ( mathrm{R}=mathrm{Me} ), the equilibrium constant ( ( mathrm{Keq} ) ) was determined to be 11 ( mathrm{M}^{-1} ). Please give the equation that would allow you to convert this Keq to ( Delta mathrm{G} ). Also, please approximate ( Delta mathrm{G} ) for this ( mathrm{Keq}=11 mathrm{M}^{-1} ).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


