Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. The rate of the following reversible gas phase reaction is said to be first order in A and first order in C and
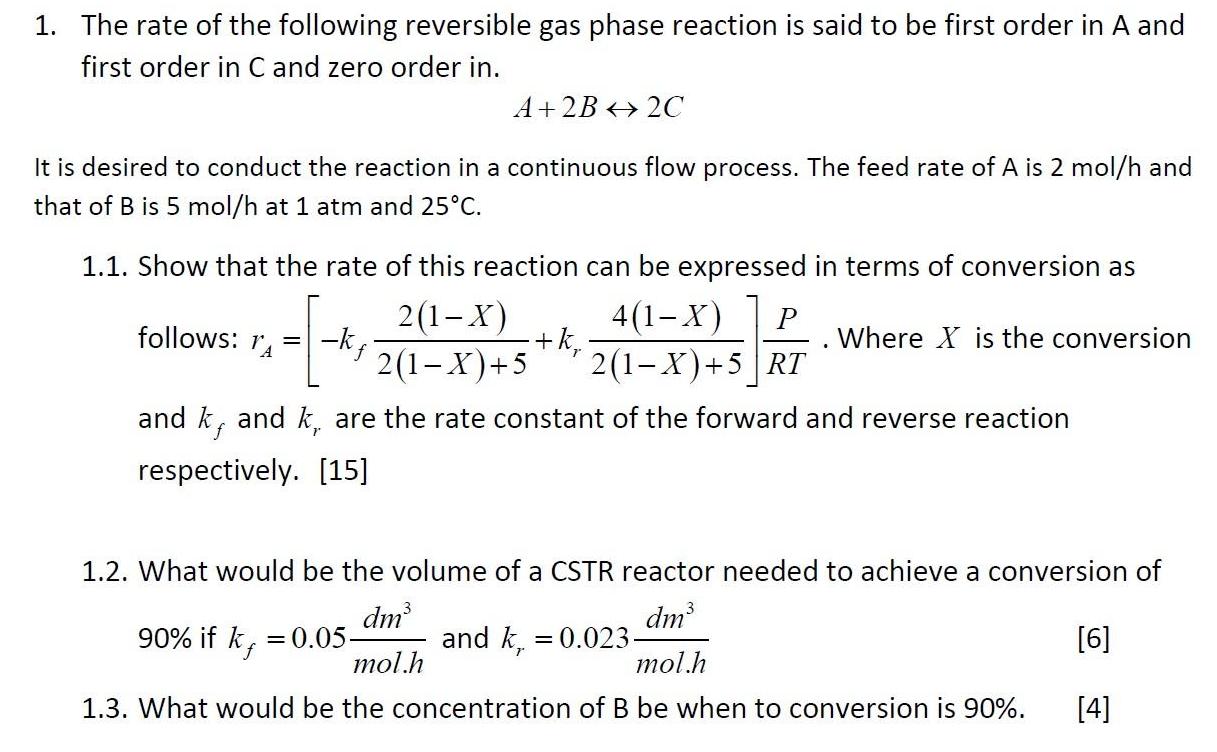
1. The rate of the following reversible gas phase reaction is said to be first order in A and first order in C and zero order in. A+2B 2C It is desired to conduct the reaction in a continuous flow process. The feed rate of A is 2 mol/h and that of B is 5 mol/h at 1 atm and 25C. 1.1. Show that the rate of this reaction can be expressed in terms of conversion as 2(1-X) 2(1-X)+5 4(1-X) +k, 2(1-X)+5 |RT follows: 1, Where X is the conversion f and k, and k, are the rate constant of the forward and reverse reaction f respectively. [15] 1.2. What would be the volume of a CSTR reactor needed to achieve a conversion of dm 90% if k, = 0.05- mol.h dm and k. = 0.023- mol.h [6] f 1.3. What would be the concentration of B be when to conversion is 90%. [4]
Step by Step Solution
★★★★★
3.46 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6362742b06091_236670.pdf
180 KBs PDF File
6362742b06091_236670.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


