Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2.1 The data in the table below was collected by sampling the contents of a batch reactor with time, where A is reacting. Assume
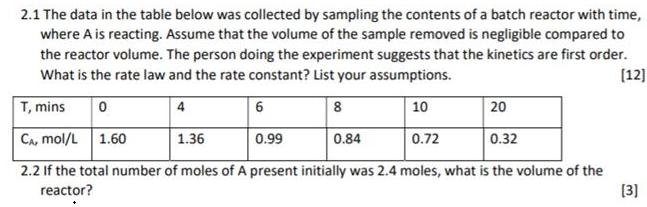
2.1 The data in the table below was collected by sampling the contents of a batch reactor with time, where A is reacting. Assume that the volume of the sample removed is negligible compared to the reactor volume. The person doing the experiment suggests that the kinetics are first order. What is the rate law and the rate constant? List your assumptions. (12] T, mins 4 6 8 10 20 CA, mol/L 1.60 1.36 0.99 0.84 0.72 0.32 2.2 If the total number of moles of A present initially was 2.4 moles, what is the volume of the [3] reactor?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
63624c2886bc1_236482.pdf
180 KBs PDF File
63624c2886bc1_236482.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


