Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question 26 26. (-/0.14 Points! DETAILS 0/4 Submissions Used Based on activation energies and energy changes and assuming that all collision factors are the same,
question 26 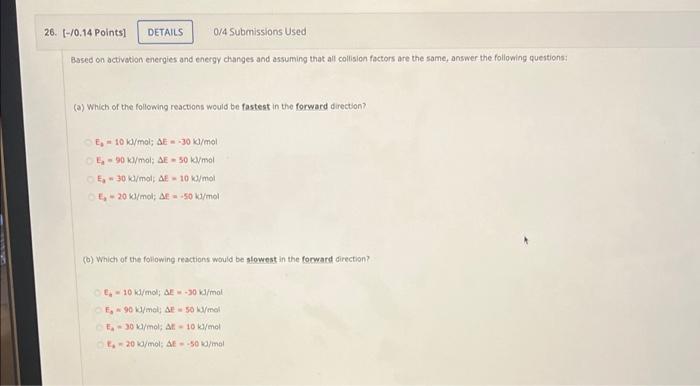
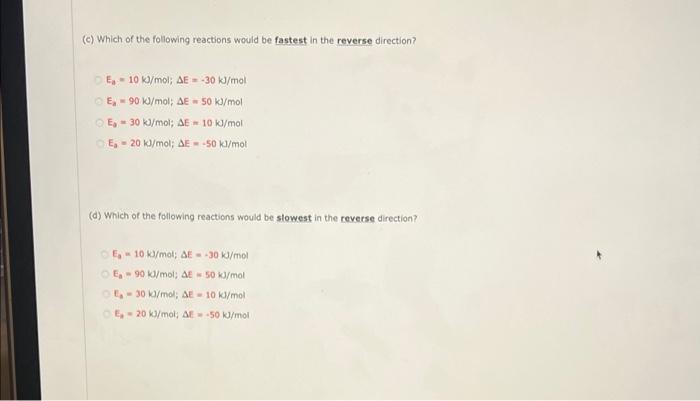
26. (-/0.14 Points! DETAILS 0/4 Submissions Used Based on activation energies and energy changes and assuming that all collision factors are the same, answer the following questions: (a) Which of the following reactions would be fastest in the forward direction 7.-10 /mol; DE -30 k/mol - 30 k/mol; AE 50 k/mol E 30 /mol AE 10 /mol - 20 l/mol; M.50 1/mol (b) Which of the following reactions would be slowest in the forward direction? - 10 kd/molE.30 /mol E90 /mol A-50 /mol t/molAL - 10 k/mol - 20 /mol: S-500/mol (c) Which of the following reactions would be fastest in the reverse direction? In - 10 J/mol; AE-30 kJ/mol E, - 90 /mol, AE = 50 kJ/mol E30 /mol; SE 10 kJ/mol E. - 20 /mol; DE -50 kl/mol (d) Which of the following reactions would be slowest in the reverse direction? 5 - 10/mol; AE-30 k/mol E - 90 l/molAE 50 kJ/mol E - 30 /mol; AE-10 k/mol E - 20 kJ/mol; AE50 kJ/mol 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


