Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 3 (2 points) A liquid mixture of 0.39 mole fraction benzene and the rest styrene at 74.3C is in equilibrium with vapours of the
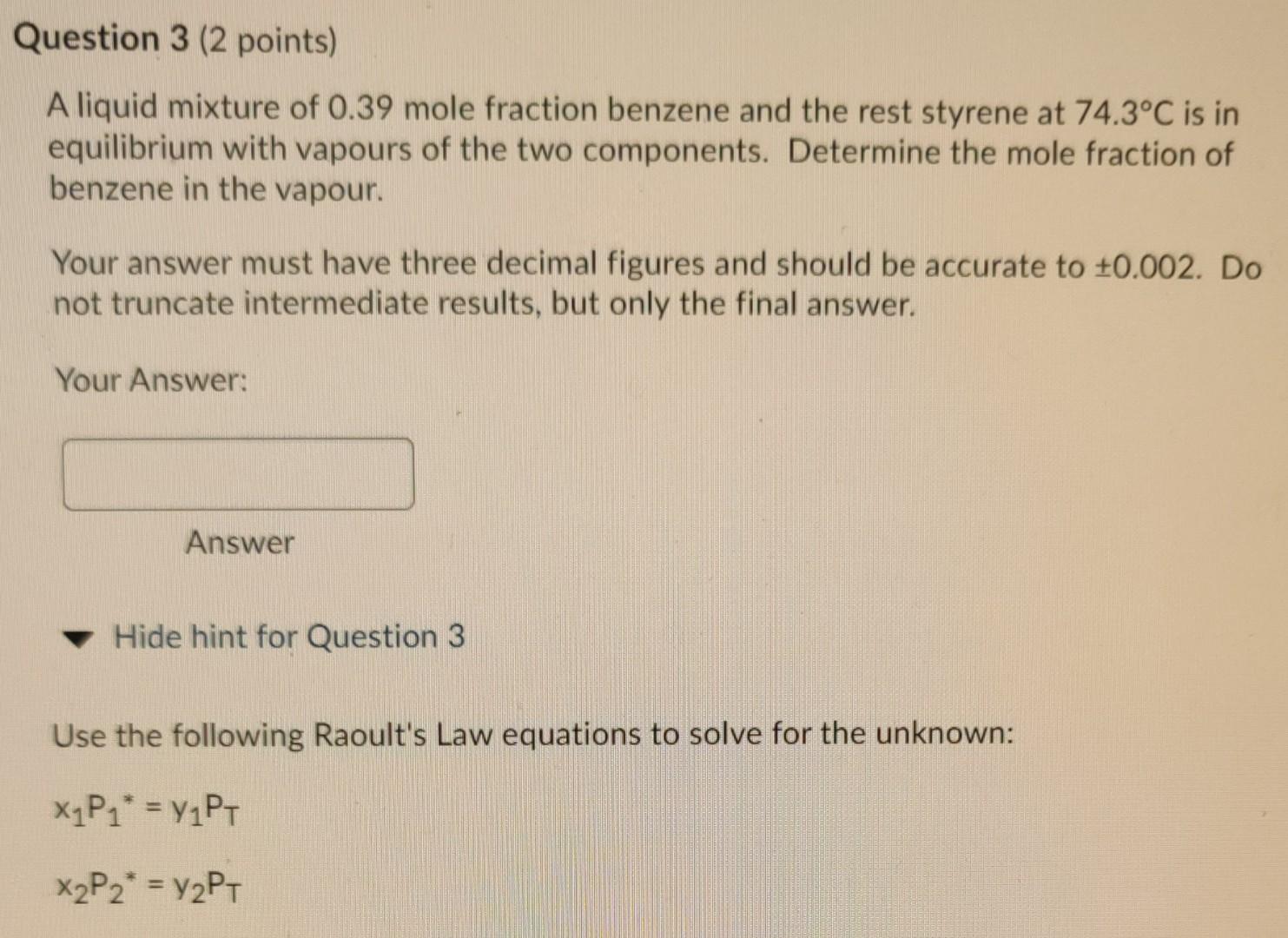

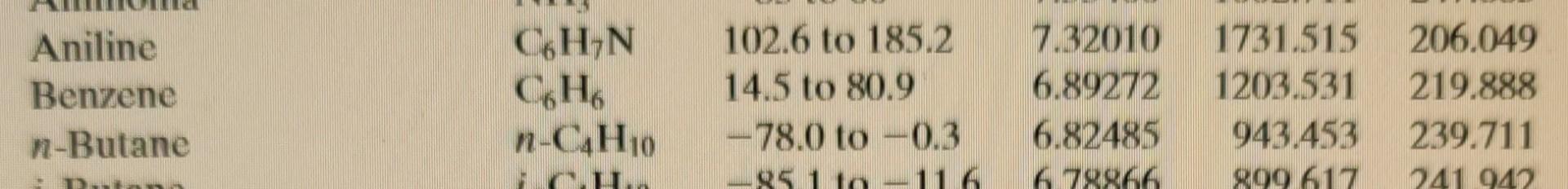
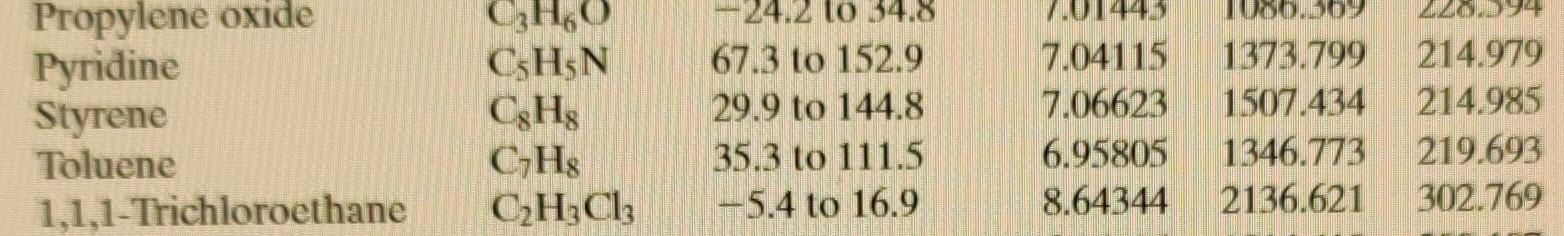
Question 3 (2 points) A liquid mixture of 0.39 mole fraction benzene and the rest styrene at 74.3C is in equilibrium with vapours of the two components. Determine the mole fraction of benzene in the vapour. Your answer must have three decimal figures and should be accurate to +0.002. Do not truncate intermediate results, but only the final answer. Your Answer: Answer Hide hint for Question 3 Use the following Raoult's Law equations to solve for the unknown: X1P1* = y1PT. X2P2* = y2PT Table B.4 (Continued) Formula Compound Range (C) B O LOUOU O 000 026 203454 Aniline Benzene n-Butane C6H,N 66 n-C4H10 102.6 to 185.2 14.5 to 80.9 -78.0 to -0.3 -95 11-116 7.32010 6.89272 6.82485 678866 1731.515 1203.531 943,453 899 617 206.049 219.888 239,711 241 940 Propylene oxide Pyridine Styrene "Toluene 1,1,1-Trichloroethane C3H60 CsHsN CgHg CHg C2H3C13 - 24.2 to 34.8 67.3 to 152.9 29.9 to 144.8 35.3 to 111.5 -5.4 to 16.9 7.04115 7.06623 6.95805 8.64344 10000 1373.799 1507.434 1346.773 2136.621 94 214.979 214.985 219.693 302.769
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


