Question: Question 5 15 pts a The force of attraction between a divalent cation and a monovalent anion is 8.02 x 10-9 N. If the ionic
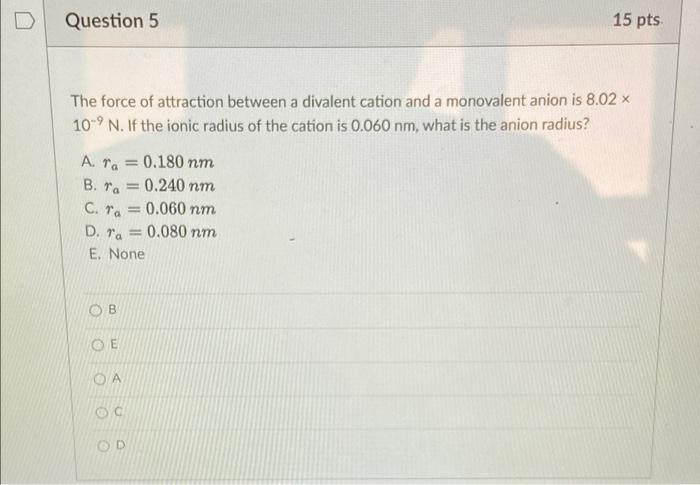
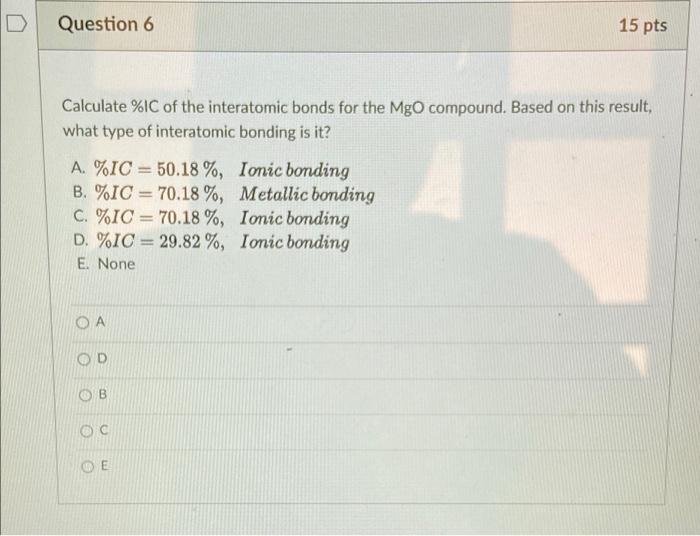
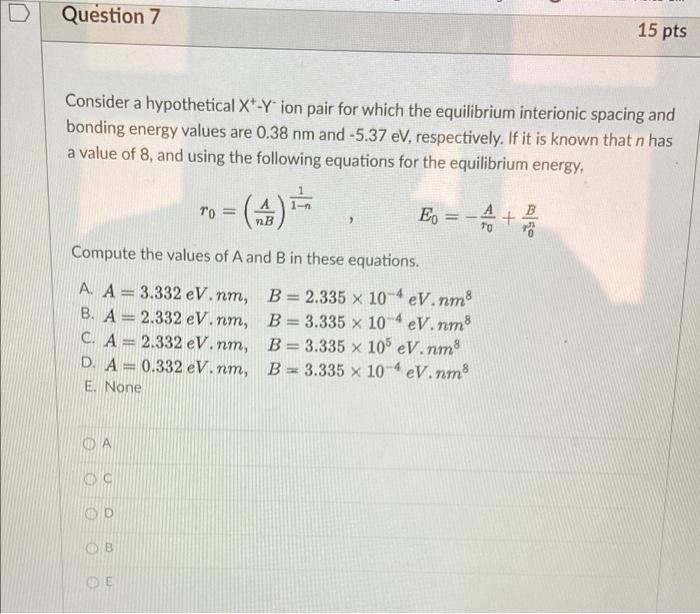
Question 5 15 pts a The force of attraction between a divalent cation and a monovalent anion is 8.02 x 10-9 N. If the ionic radius of the cation is 0.060 nm, what is the anion radius? Ara 0.180 nm B. ra=0.240 nm C. ra = 0.060 nm D. ra = 0.080 nm E. None B OD Question 6 15 pts Calculate %IC of the interatomic bonds for the MgO compound. Based on this result, what type of interatomic bonding is it? A. %IC = 50.18%, Ionic bonding B. %IC = 70.18%, Metallic bonding C. %IC = 70.18%, Ionic bonding D. %IC = 29.82 %, Ionic bonding E. None OE Question 7 15 pts Consider a hypothetical X+-Y ion pair for which the equilibrium interionic spacing and bonding energy values are 0.38 nm and -5.37 eV, respectively. If it is known that n has a value of 8, and using the following equations for the equilibrium energy, To = - ( nB E B + Compute the values of A and B in these equations. A. A = 3.332 eV nm, B. A = 2.332 eV.nm, C. A = 2.332 eV.nm, D. A=0.332 eV.nm, E None B = 2.335 x 10-4 eV nm B = 3.335 x 10-4 eV.nm8 B = 3.335 x 10 eV.nm8 B = 3.335 x 10-4 eV.nm8 D A OD B OE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


