Question
Question: In the BETT theory of colloids, a certain colloid system exhibits monolayer adsorption of gas molecules on the surface of the colloidal particles.
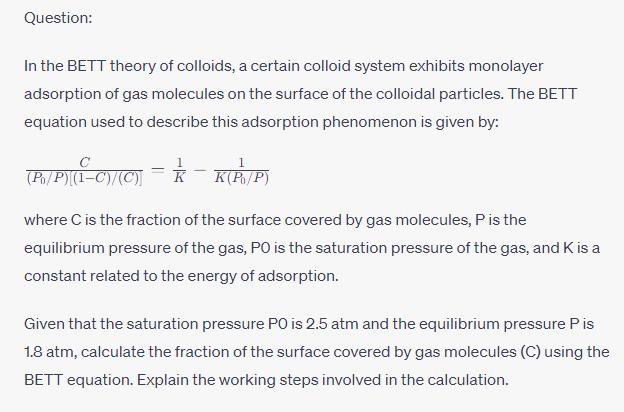
Question: In the BETT theory of colloids, a certain colloid system exhibits monolayer adsorption of gas molecules on the surface of the colloidal particles. The BETT equation used to describe this adsorption phenomenon is given by: - (P./P) (1-C)/(C) = -K(P/P) where C is the fraction of the surface covered by gas molecules, P is the equilibrium pressure of the gas, PO is the saturation pressure of the gas, and K is a constant related to the energy of adsorption. Given that the saturation pressure PO is 2.5 atm and the equilibrium pressure P is 1.8 atm, calculate the fraction of the surface covered by gas molecules (C) using the BETT equation. Explain the working steps involved in the calculation.
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



