
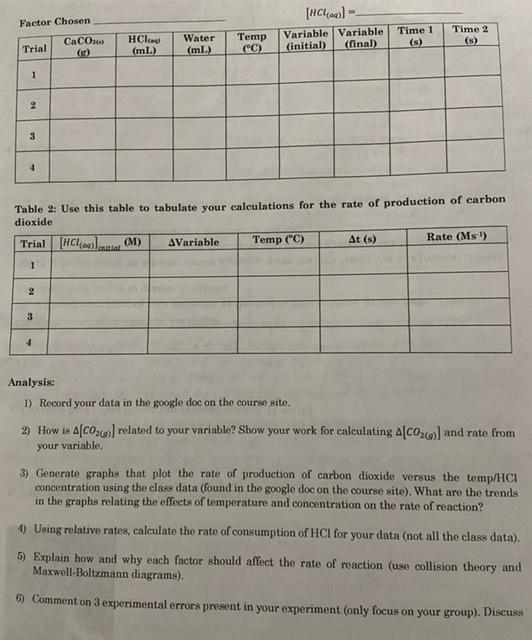
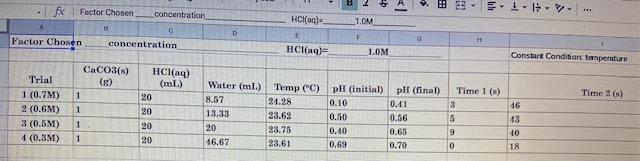
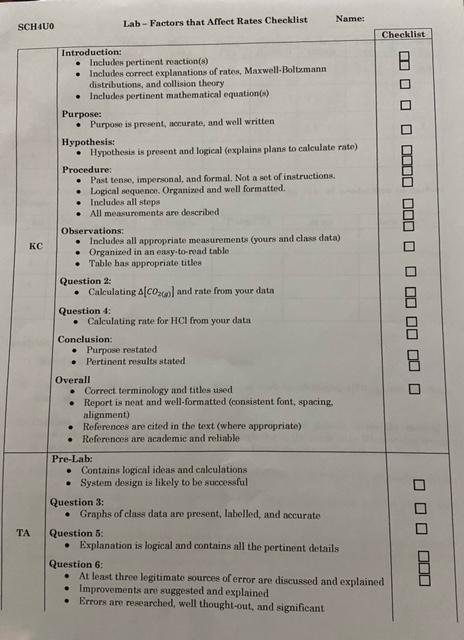
SCHUO Lab-Factors That Affect Rates Name: For the following experiment you are going to test how temperature and concentration impact the rate of a chemical reaction. We are going to test the reaction of calcium carbonate with hydrochloric acid, CaCO3+2 HCL) +CaCl + CO2(0)+H To determine the rate of reaction you will have to detect a change in the amount of one substance so that you can calculate a rate. To do this you will have access to the advanced chemistry sensor (which can measure pH or temperature), mas balances, hot plates, and ice. Each group will need to choose a set of probe to use and create their own procedure for this. Reminder: CV GV GV where is concentration in mol/l and V is volume measured in L. Your group will choose ONE factor to work on (temp. or concentration) and ONE variable to measure to determine the rate of reaction (temperature, pH, mass, etc...). Instructions: 1) Pair the probe to your ipad and open the sparkvue app. You can setup the app to graph pH temperature versus time. You can also have it display multiple variables simultaneously if you would like 2) Obtain 200.0 mL of 1.0 M hydrochloric acid as a stock solution 3) If you are testing temperature, heatcool your HCI it must be below 50C) and combine with your calcium carbonate. 4) If you are testing concentration, mix your HCI with water to create solutions with varying concentration and combine them with calcium carbonate when ready. 5) Waste can be washed down the sink. 6) Repeat your procedure multiple times varying either the temperature of the system or the ratio of HCl to water. Use hot/cold water baths to manipulate the temperature. 7) Write your results on the board for the class to use Pre-Lab: You nre going to have to do some calculations and planning beforehand to ensure that everything goes smoothly during the experiment. Make sure that you decide on and/or calculate the amounts of the following materials that you will need: Calcium carbonate mass . 1.0 M HCl volume for each trial Limiting reagent Which variable you are mensuring to determine rate How to calculate the rate of production of carbon dioxide using the measured variable Observations: Record your observations in the table(s) below. Please note that there should be actual measured numbers and should be different from your planned amount. You should also ensure that you measure the proper number of significant digits. Factor Chosen [HCl(0) Variable Variable (initial) (final) Time 1 CaCO3 HCl (mL) Trial Water (ml) Time 2 (S) Temp CC) 1 2 3 4 Table 2: Use this table to tabulate your calculations for the rate of production of carbon dioxide Trial [HCl(aq)] (M) AVariable Temp (C) At (8) Rate (Ms) 1 Jimi 2 3 4 Analysis: 1) Record your data in the google doc on the course site. 2) How is AC0360) related to your variable? Show your work for calculating 4C06) and rate from your variable 3) Generate graphs that plot the rate of production of carbon dioxide versus the temp/HCI concentration using the class data (found in the google doc on the course site). What are the trends in the graphs relating the effects of temperature and concentration on the rate of reaction? 4) Using relative rates, calculate the rate of consumption of HCl for your data (not all the class data). 5) Explain how and why each factor should affect the rate of reaction (use collision theory and Maxwell-Boltzmann diagrams). 6) Comment on 3 experimental errors present in your experiment (only focus on your group). Discuss Factor Chosen IV. 1 11. concentration 1.0M 8 HCl(0) E HCl(aq) Factor Chosen F H concentration 1.OM Constant Conditions temperature CaCO3(s) (2) Water (ml.) 1 Time 2) Trial 1 (0.7M) 2 (0.6M) 3 (0.5M) 1 (0.3M) HCl(aq) (mL) 20 20 20 20 1 Temp (C) pH (initial) pH (final) 24.28 0.10 0.41 23.62 0.50 0.56 23.75 0.40 0.65 23.61 0.69 0.70 Time 1(s) 3 5 8.57 13.33 20 46.67 1 1 46 43 10 18 9 0 SCHUO Name: Lab-Factors that Affect Rates Checklist Checklist BILD Introduction: Includes pertinent reaction() Includes correct explanations of rates, Maxwell-Boltzmann distributions, and collision theory Includes pertinent mathematical equation() Purpose: Purpose is present, accurate, and well written Hypothesis: Hypothesis is present and logical (explains plans to calculate rate) Procedure: Past tense, impersonal, and formal. Not a set of instructions Logical sequence. Organized and well formatted, Includes all steps All measurements are described Observations: Includes all appropriate measurements (yours and class data) Organized in an esy.to-read table Table has appropriate titles Question 2: Calculating Co. and rate from your data Question 4 Calculating rate for HCl from your data Conclusion: Purpose restated Pertinent results stated 0000 O Overall Correct terminology and titles used Report is neat and well-formatted (consistent font, spacing. alignment) References are cited in the text (where appropriate) References are academic and reliable TA Pre-Lab: Contains logical ideas and calculations System design is likely to be successful Question 3: Graphs of class data are present, Inbelled, and accurate Question : Explanation is logical and contains all the pertinent details Question 6: At least three legitimate sources of error are discussed and explained Improvements are suggested and explained Errors are researched, well thought-out, and significant BU










