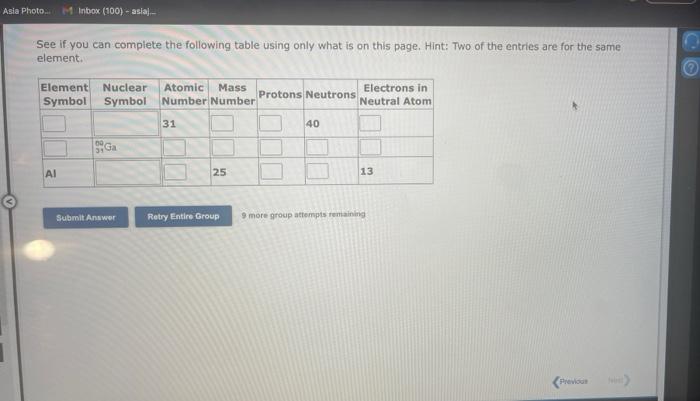
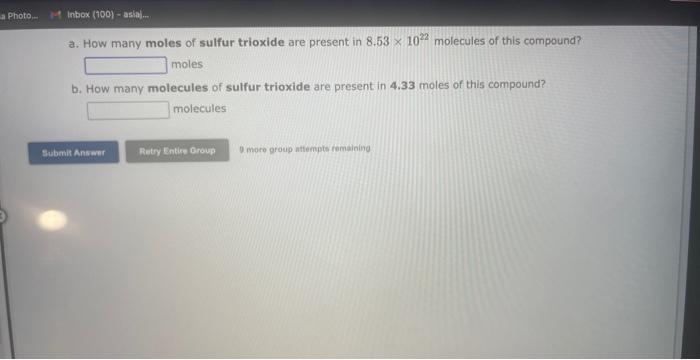
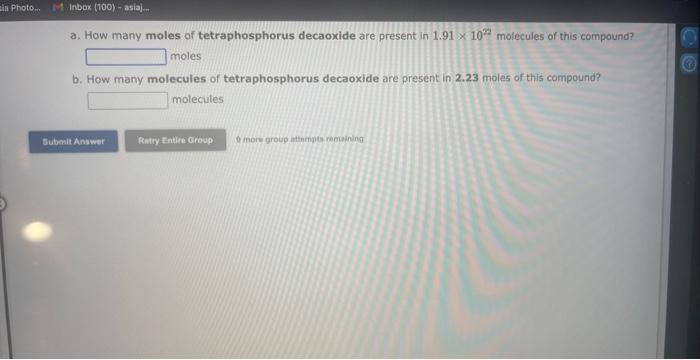
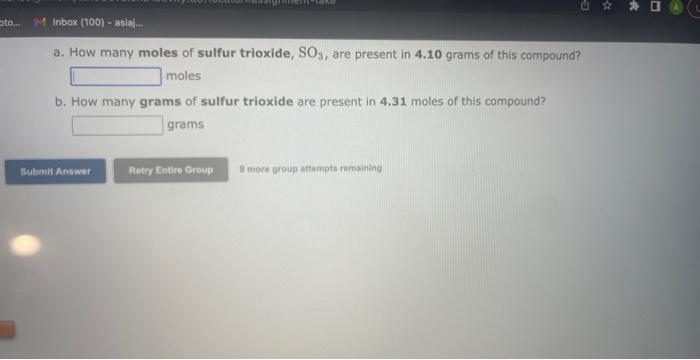
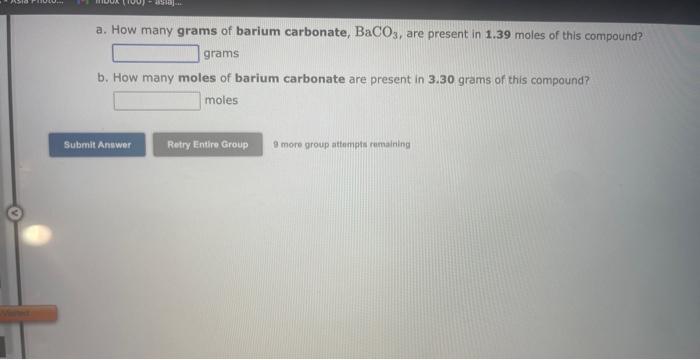
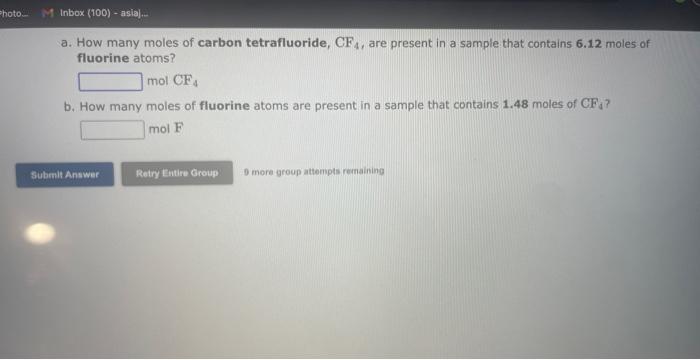
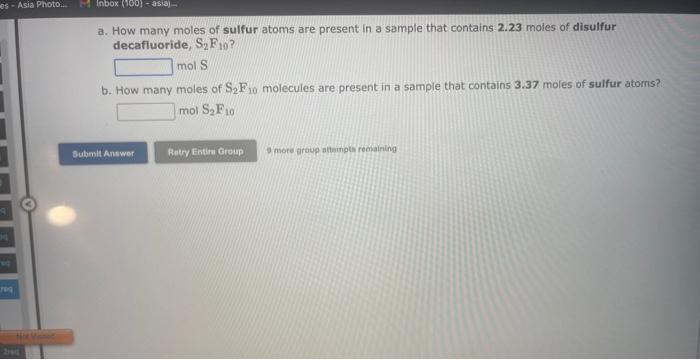
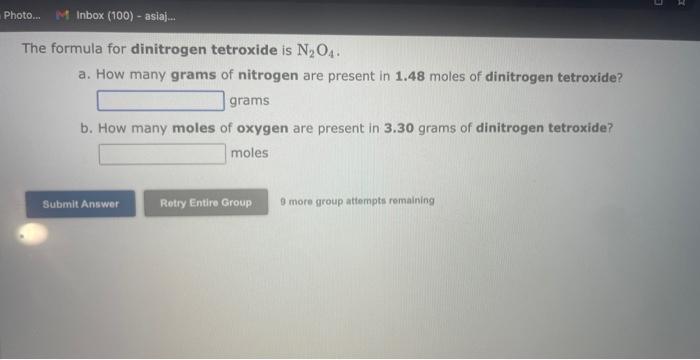
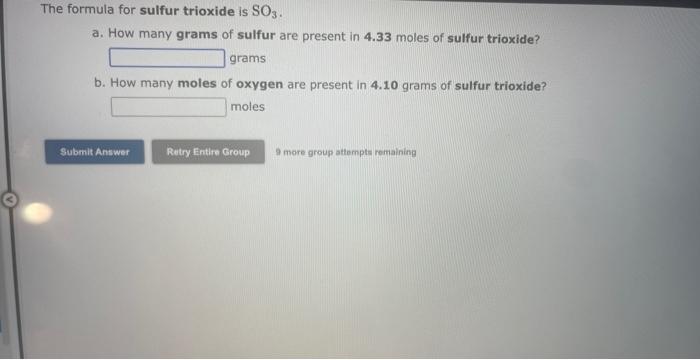
See if you can complete the following table using only what is on this page. Hint: Two of the entries are for the same element. 9 more gracip attempls temaining a. How many moles of sulfur trioxide are present in 8.531022 molecules of this compound? moles b. How many molecules of sulfur trioxide are present in 4.33 moles of this compound? molecules 9. mure eroup atfempts femaining a. How many moles of tetraphosphorus decaoxide are present in 1.911022 molecules of this compound? moles b. How many molecules of tetraphosphorus decaoxide are present in 2.23 moles of this compound? molecules 0 inare group mifnetipts remaining a. How many moles of sulfur trioxide, SO3, are present in 4.10grams of this compound? moles b. How many grams of sulfur trioxide are present in 4.31 moles of this compound? grams 9 more group attempts remaining a. How many grams of barium carbonate, BaCO3, are present in 1.39 moles of this compound? grams b. How many moles of barium carbonate are present in 3.30 grams of this compound? moles 9 more group attempts remaining a. How many moles of carbon tetrafluoride, CF4, are present in a sample that contains 6.12 moles of fluorine atoms? mol CF4 b. How many moles of fluorine atoms are present in a sample that contains 1.48 moles of CF4 ? mol F 9 more group attempts remaining a. How many moles of sulfur atoms are present in a sample that contains 2.23 moles of disulfur decafluoride, S2F10 ? mols b. How many moles of S2F10 molecules are present in a sample that contains 3.37 moles of sulfur atoms?: molS2F10 3 nsore group atteingtin reciaining The formula for dinitrogen tetroxide is N2O4. a. How many grams of nitrogen are present in 1.48 moles of dinitrogen tetroxide? grams b. How many moles of oxygen are present in 3.30 grams of dinitrogen tetroxide? moles 8 more group attempts remaining The formula for sulfur trioxide is SO3. a. How many grams of sulfur are present in 4.33 moles of sulfur trioxide? grams b. How many moles of oxygen are present in 4.10grams of sulfur trioxide? moles 9 more group attempta rettaining