Question: Select the oxidizing or reducing agent(s) that you would use to carry out the transformation below. OH HC i. 03, CH3OH ii. (CH3)2S PCC
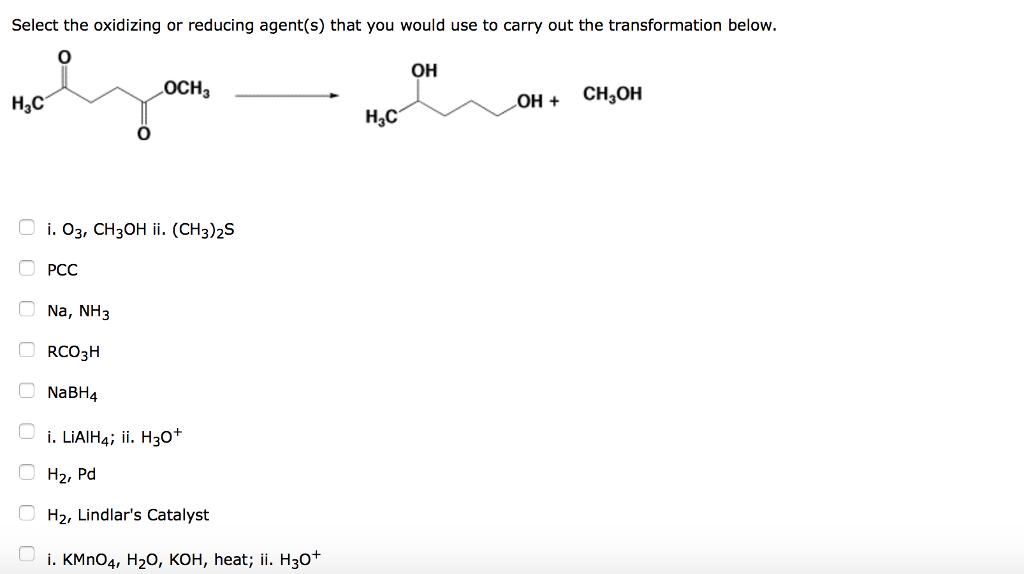

Select the oxidizing or reducing agent(s) that you would use to carry out the transformation below. OH HC i. 03, CH3OH ii. (CH3)2S PCC Na, NH3 ORCO3H NaBH4 LOCH3 i. LiAlH4; ii. H3O+ H, Pd H, Lindlar's Catalyst i. KMnO4, HO, KOH, heat; ii. H3O+ HC OH + CHOH Select the oxidizing or reducing agent(s) that you would use to carry out the transformation below.
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
In this reaction the alcohols are convertd oxidized to aldehydes So ... View full answer

Get step-by-step solutions from verified subject matter experts


