Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show the steps in detail thank you so much :) Ammonia has been studied as an alternative clean fuel for internal combustion engines, since its
show the steps in detail thank you so much :) 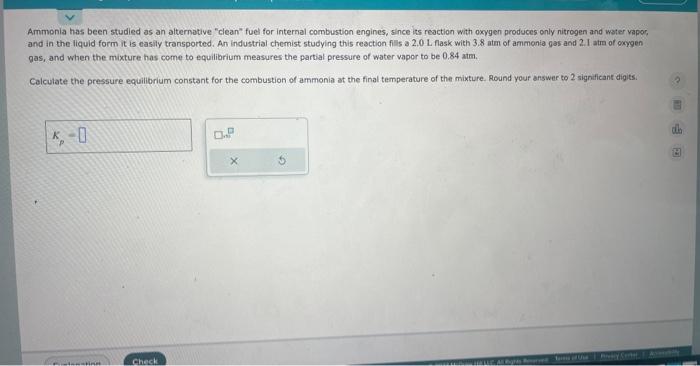
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily tronsported. An industrial chemist studying this reaction fills a 2.0L flask with 3.8 atm of ammonia gas and 2.I atra of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of water vapor to be 0.84 atm. Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


