Answered step by step
Verified Expert Solution
Question
1 Approved Answer
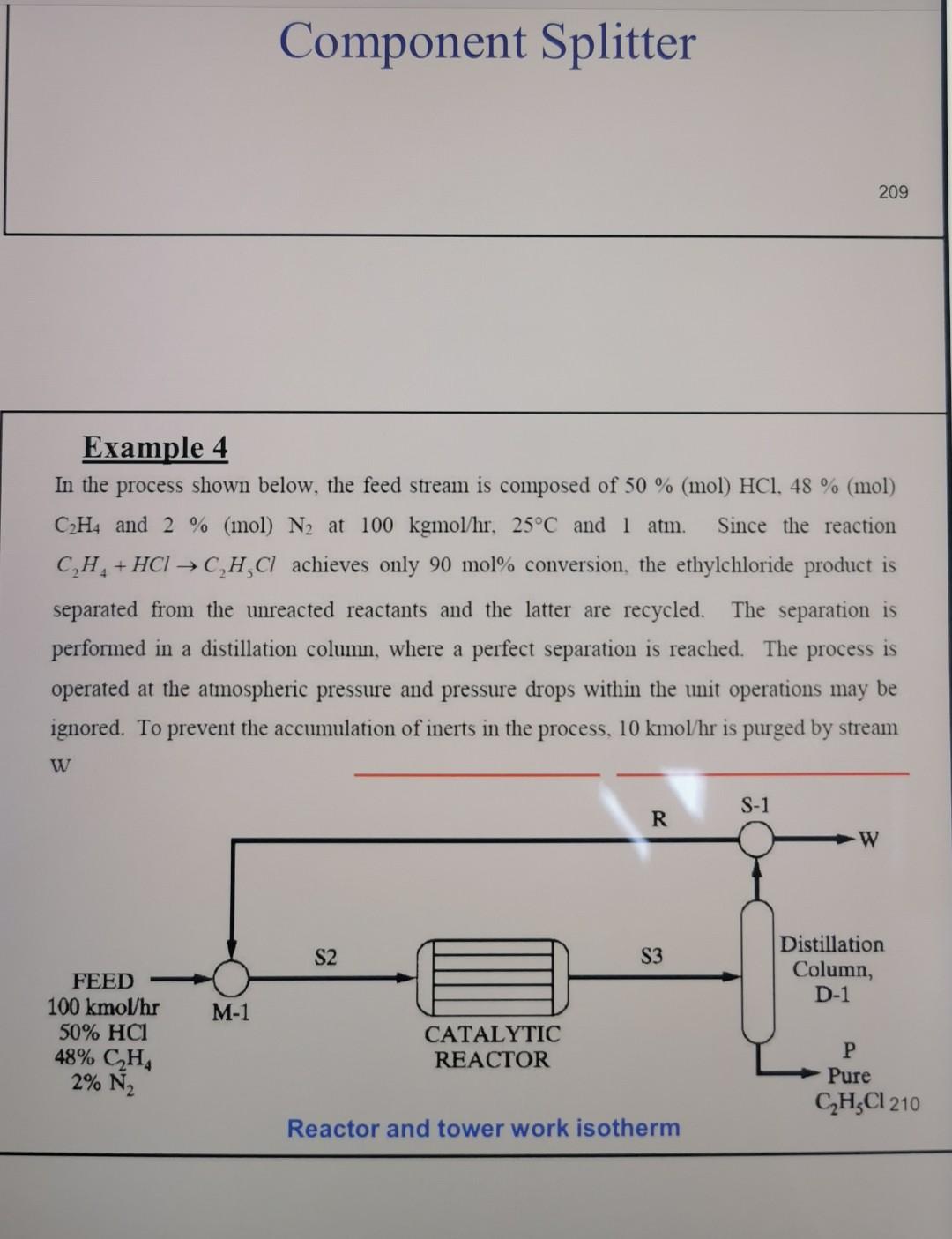
Solution Introduction Example 4 In the process shown below, the feed stream is composed of 50% (mol) HCl, 48% (mol) C2H4 and 2% (mol) N2
Solution
Introduction
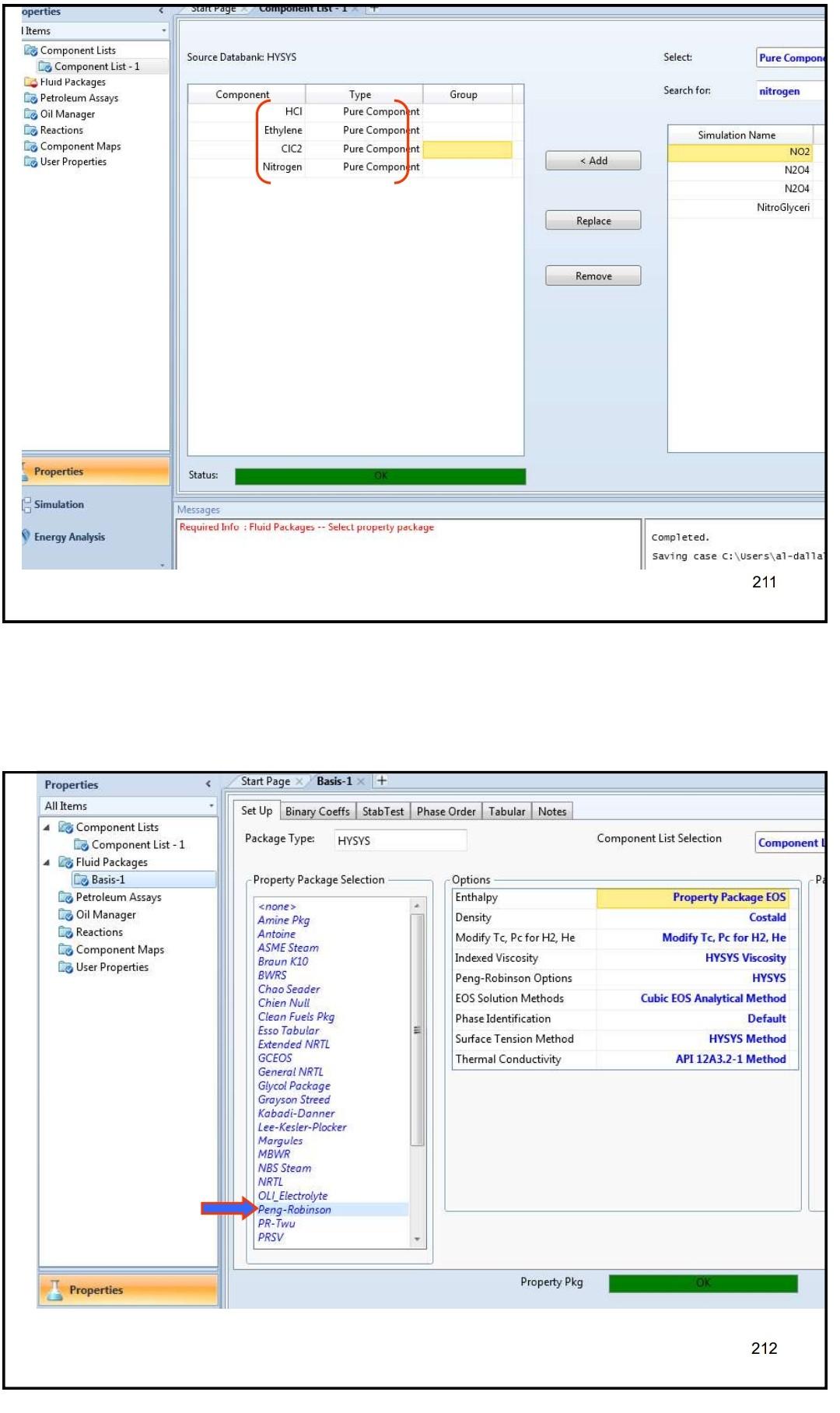
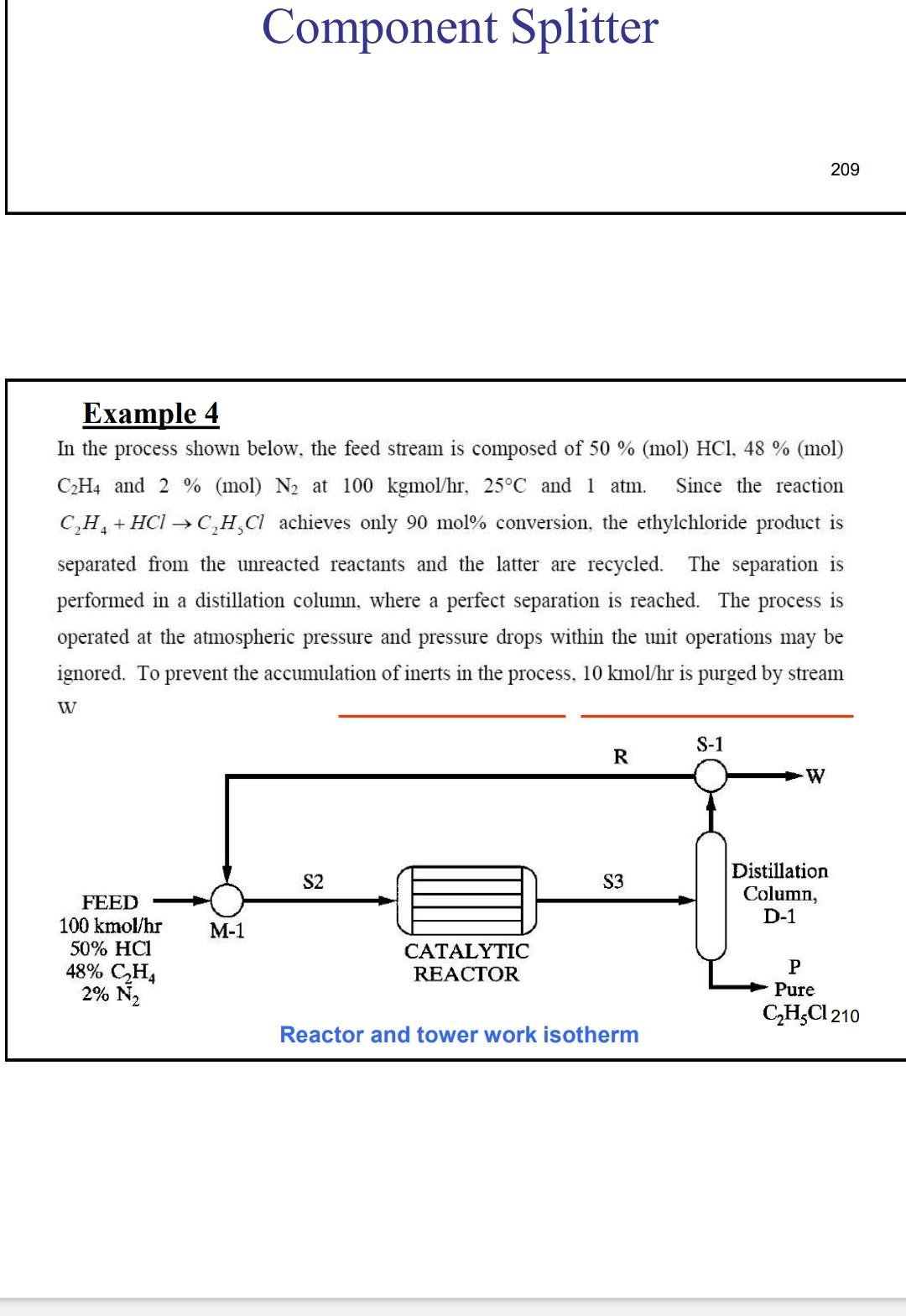
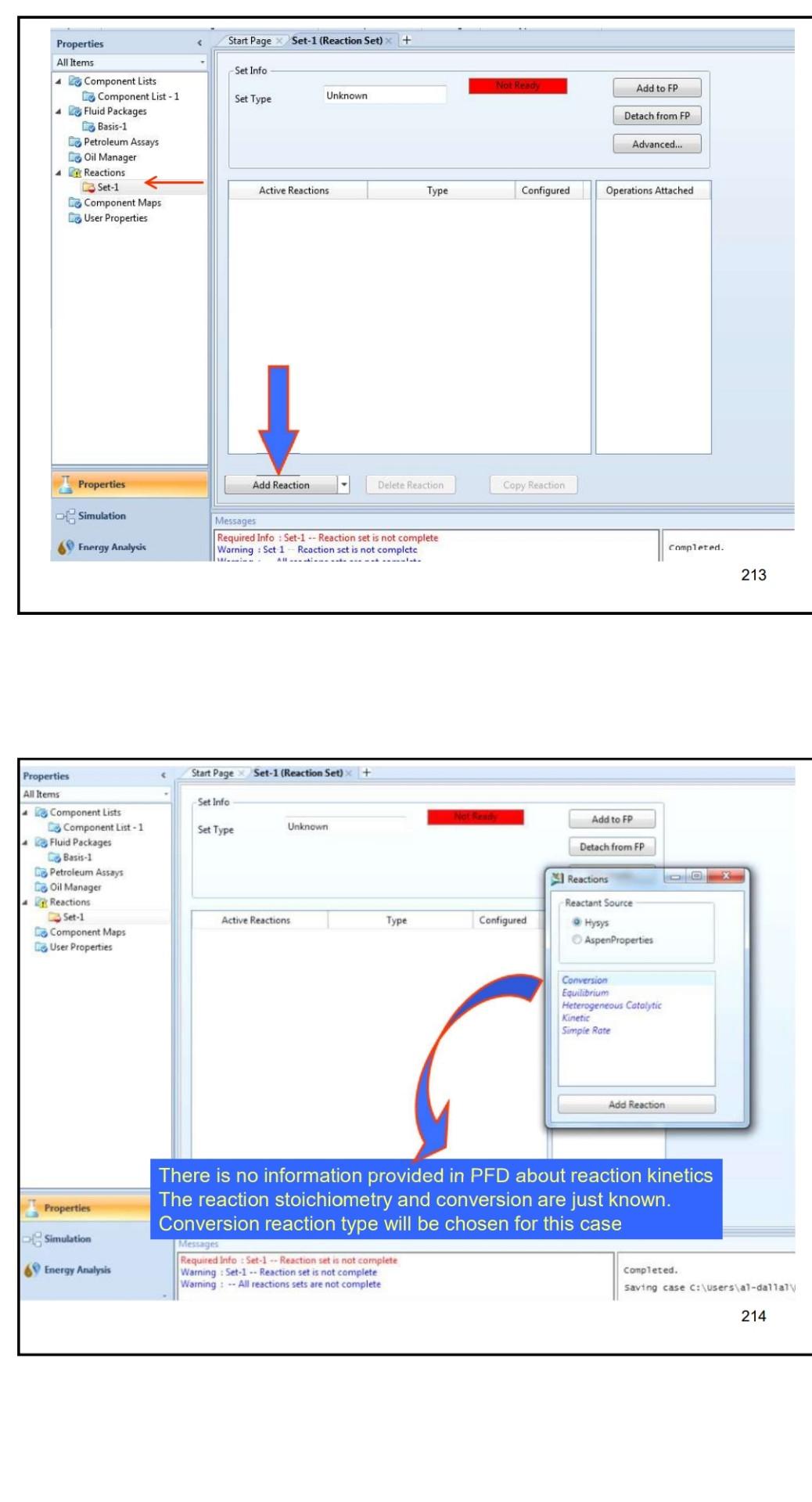
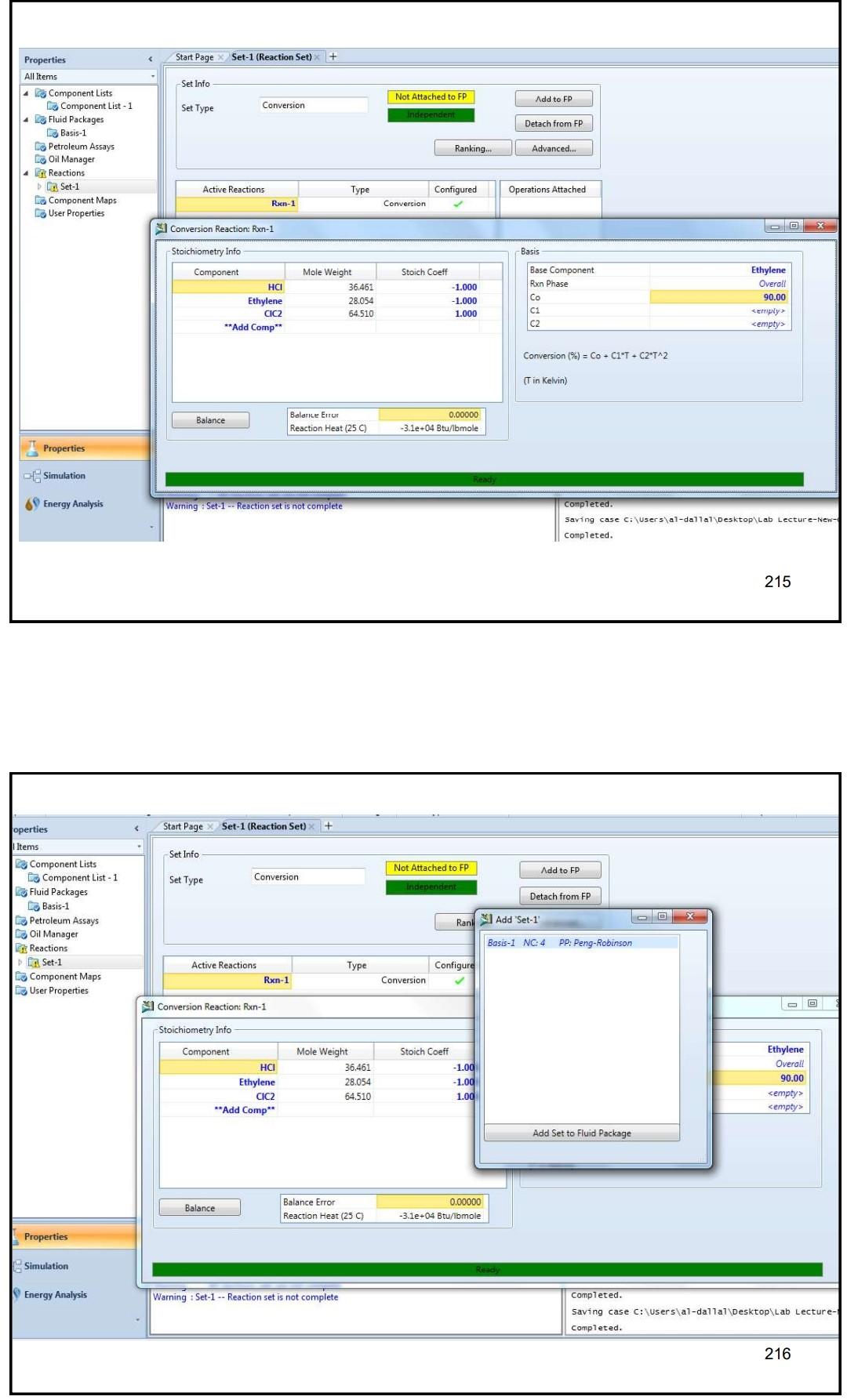
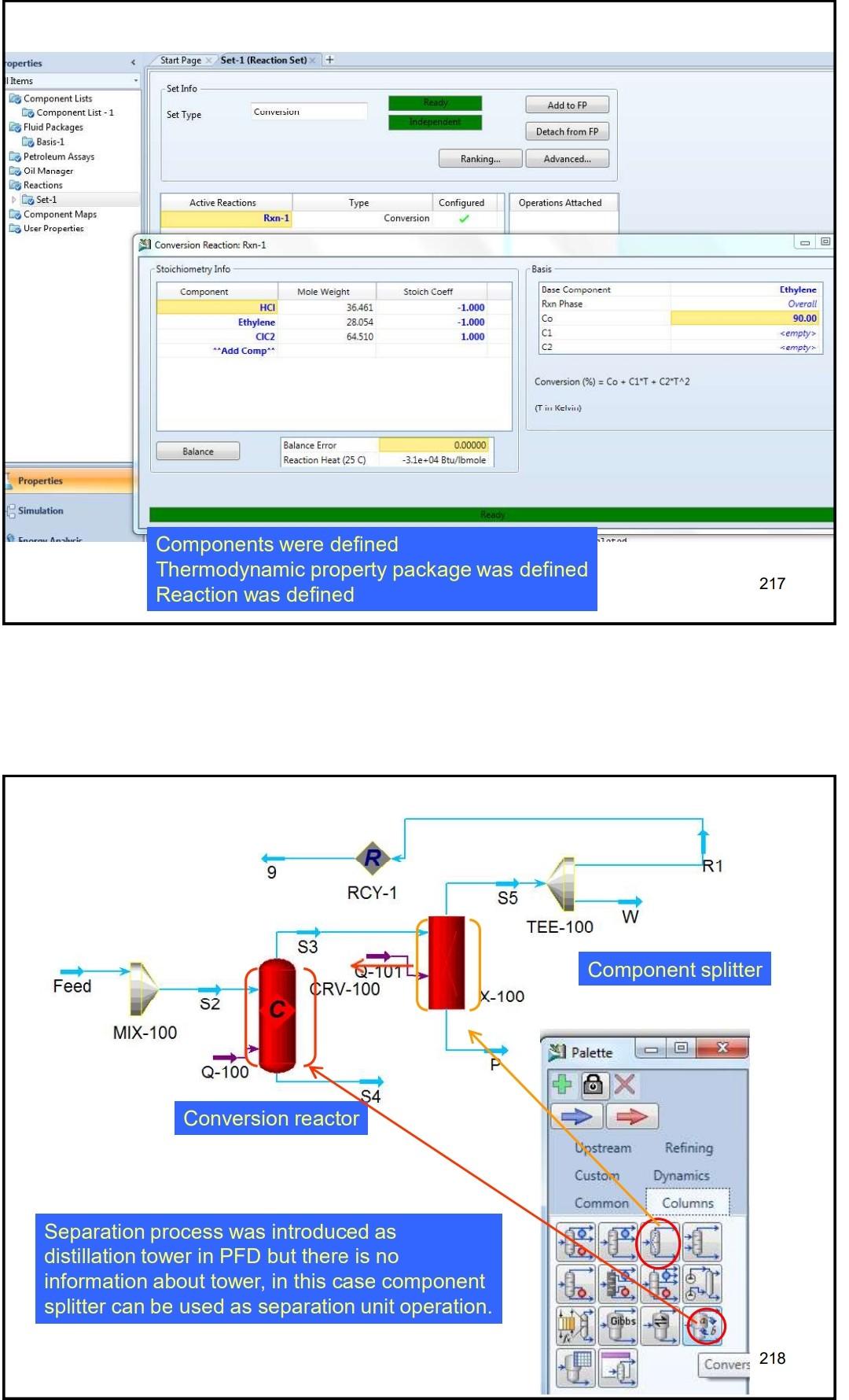
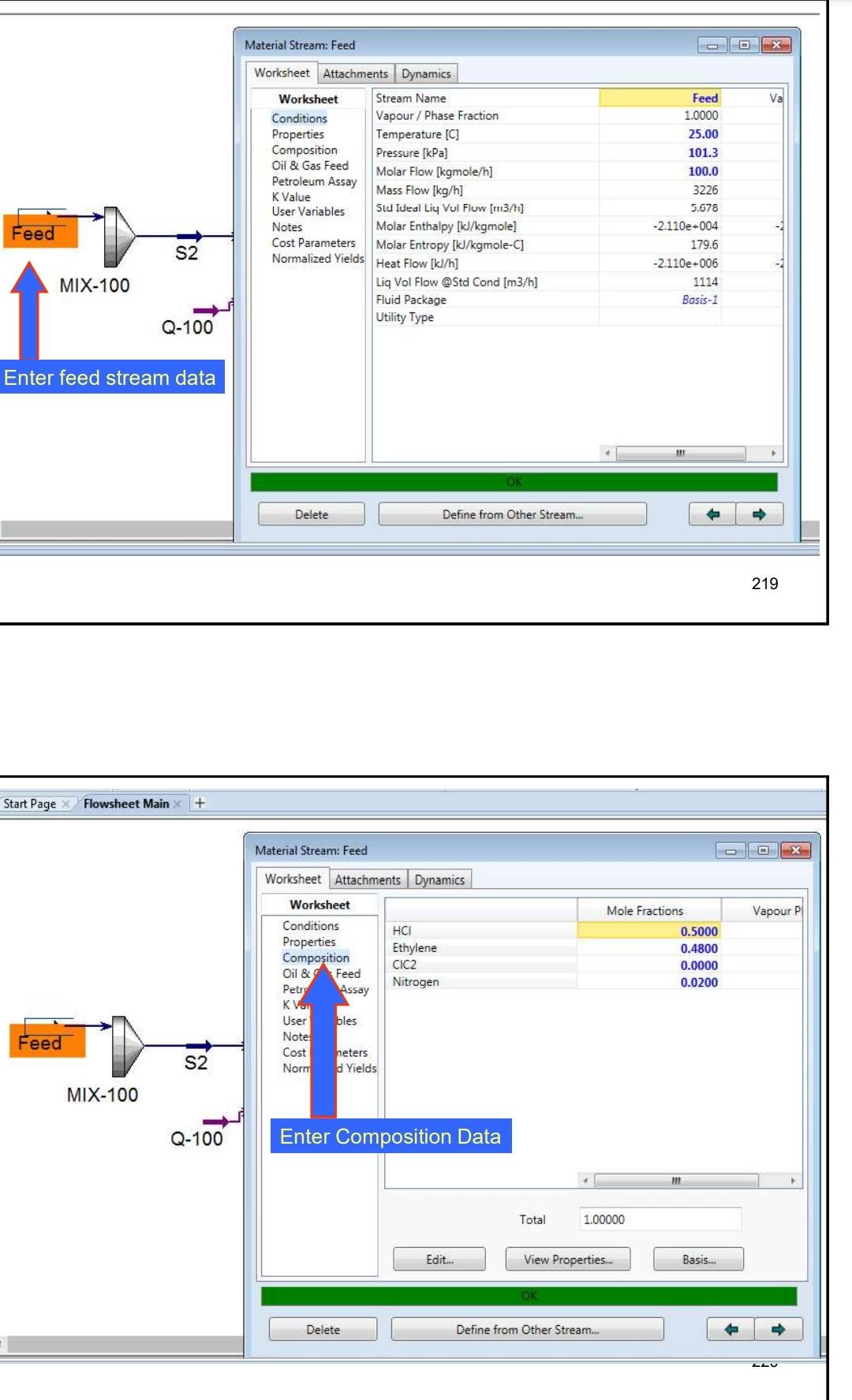
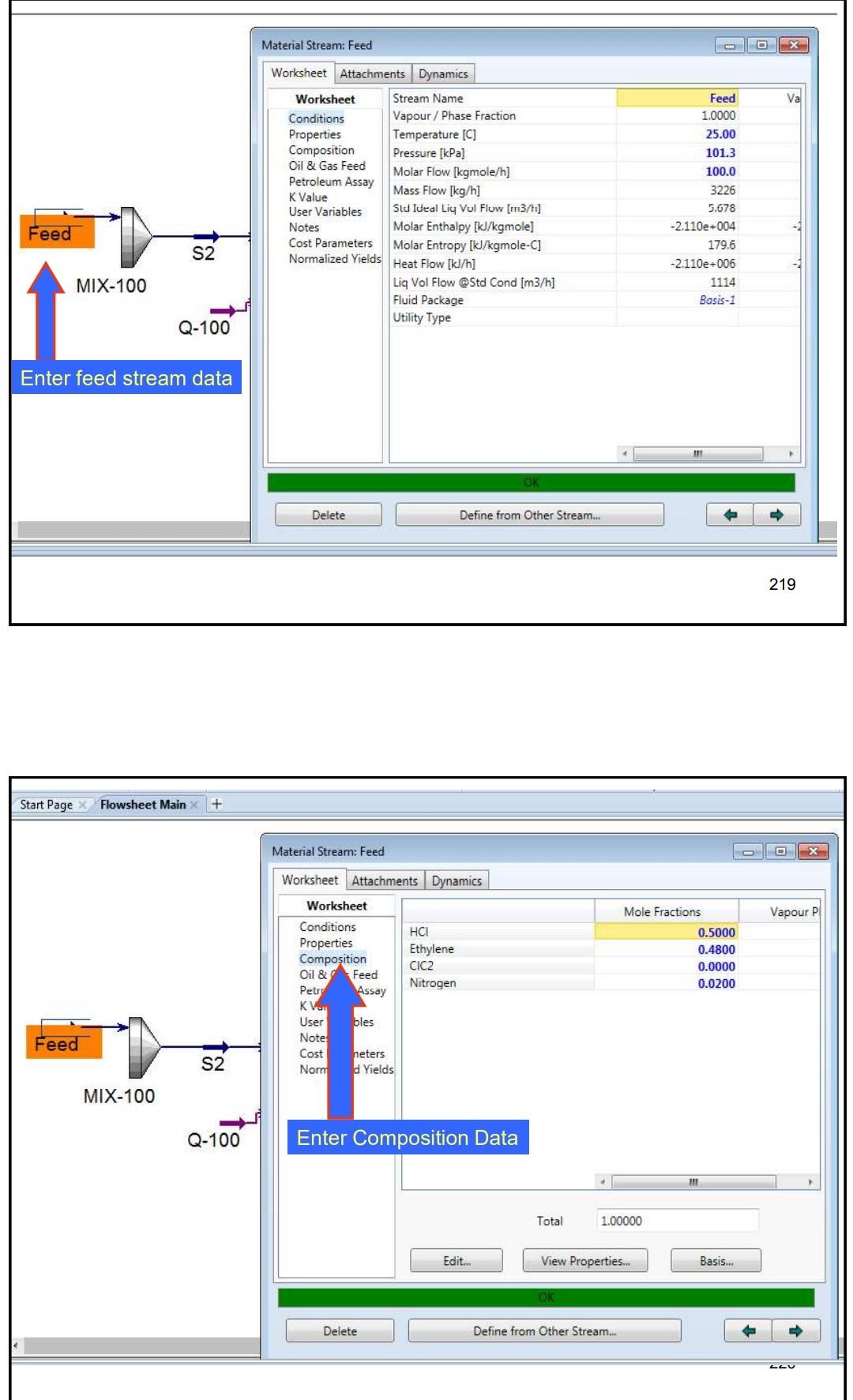
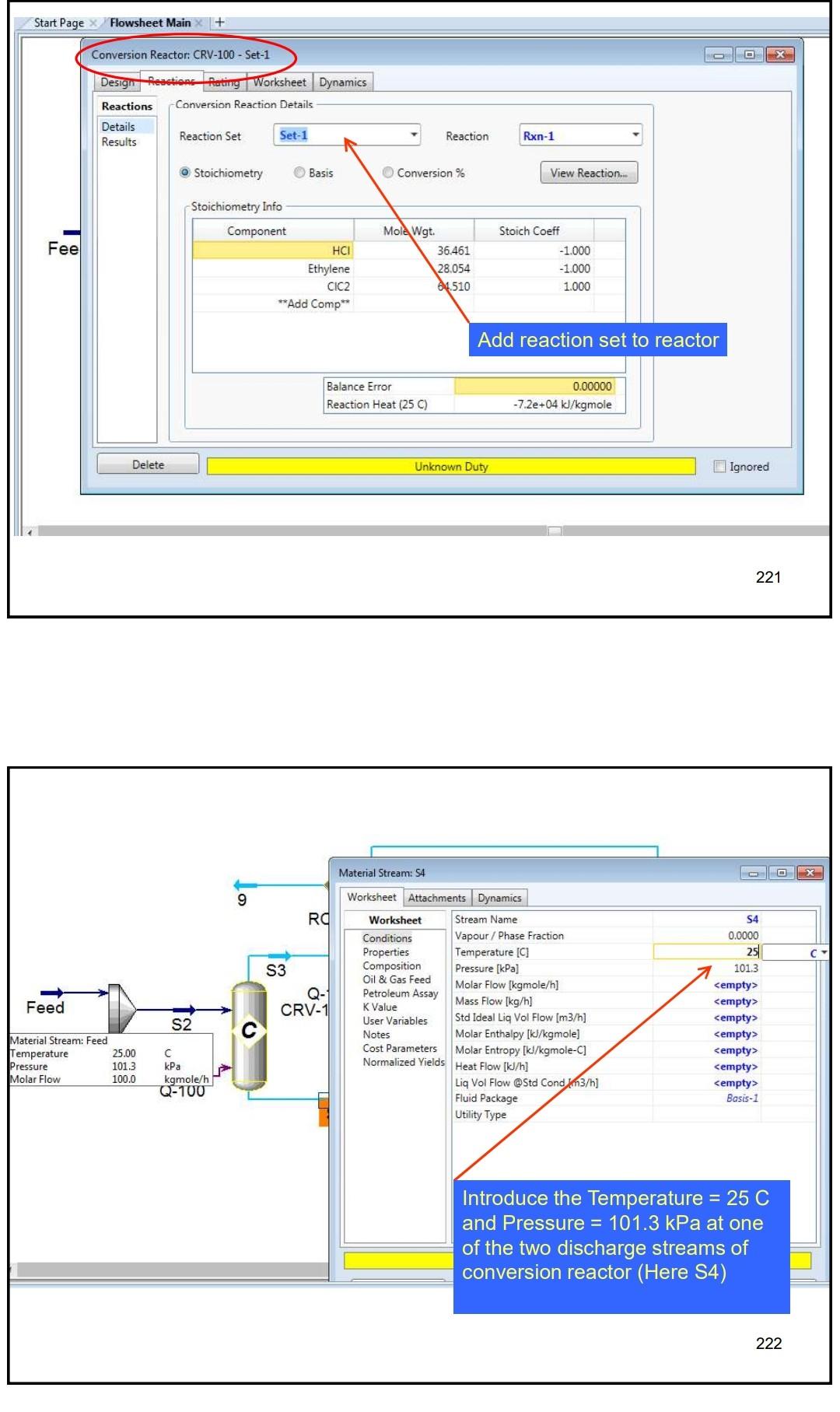
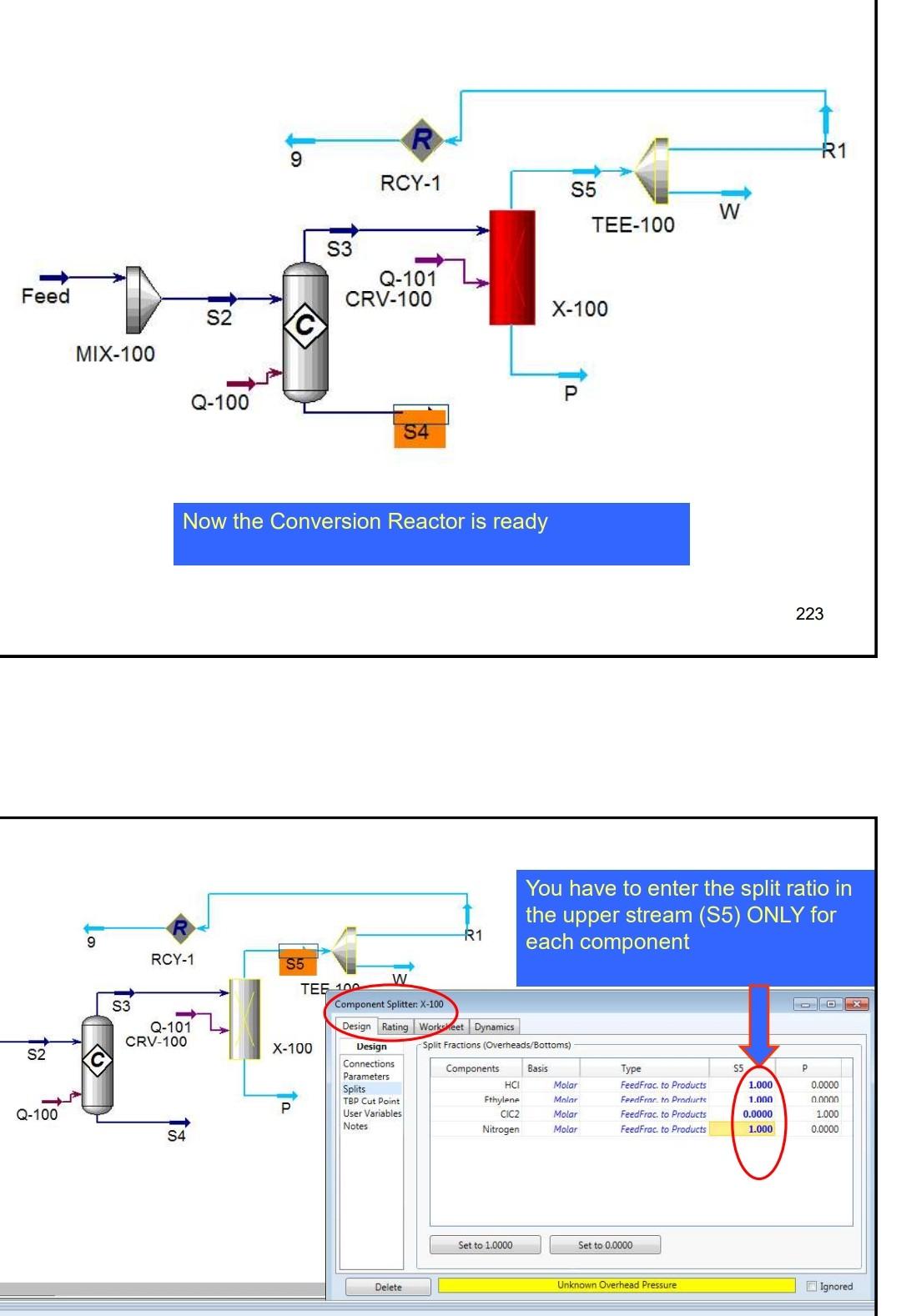
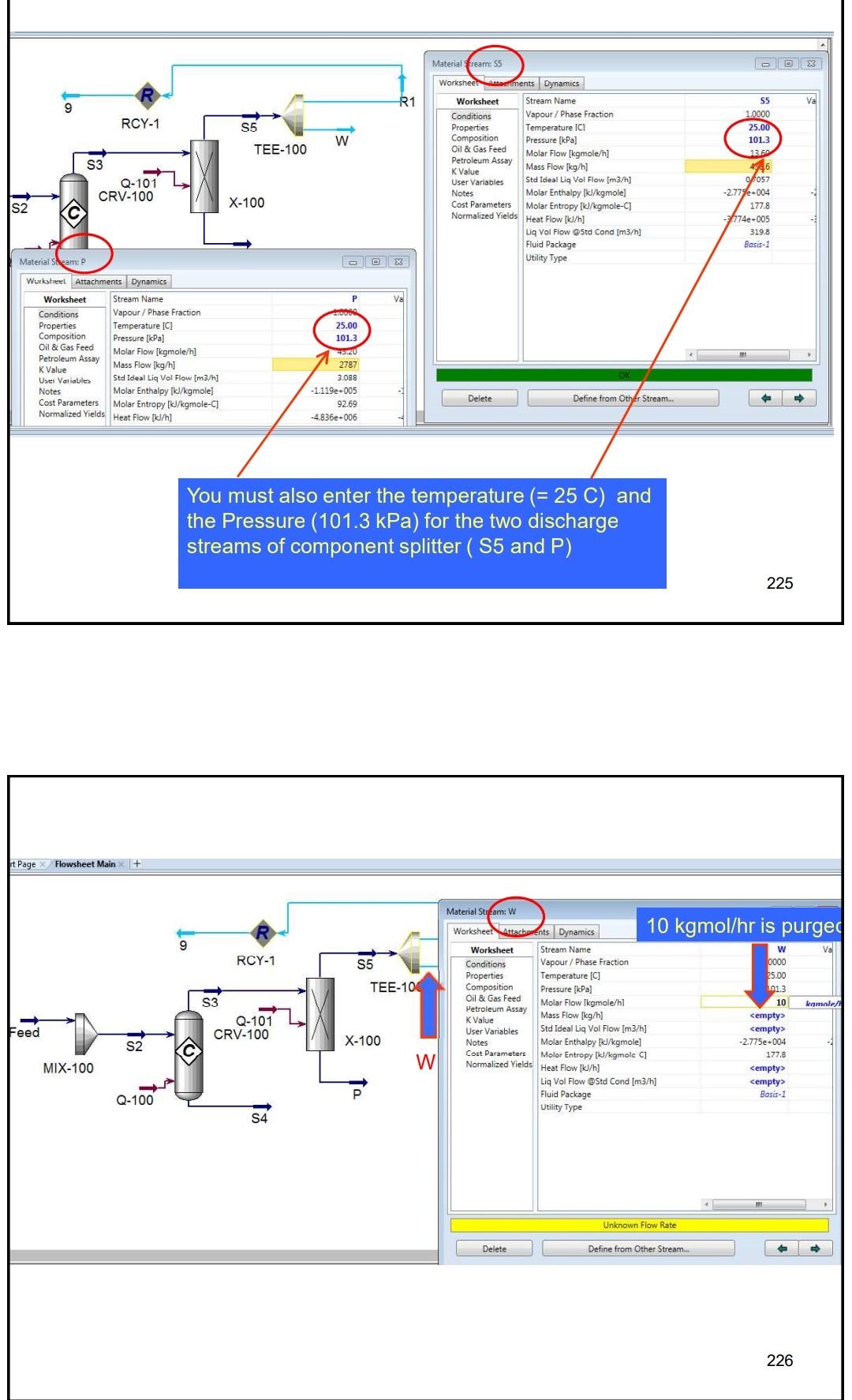
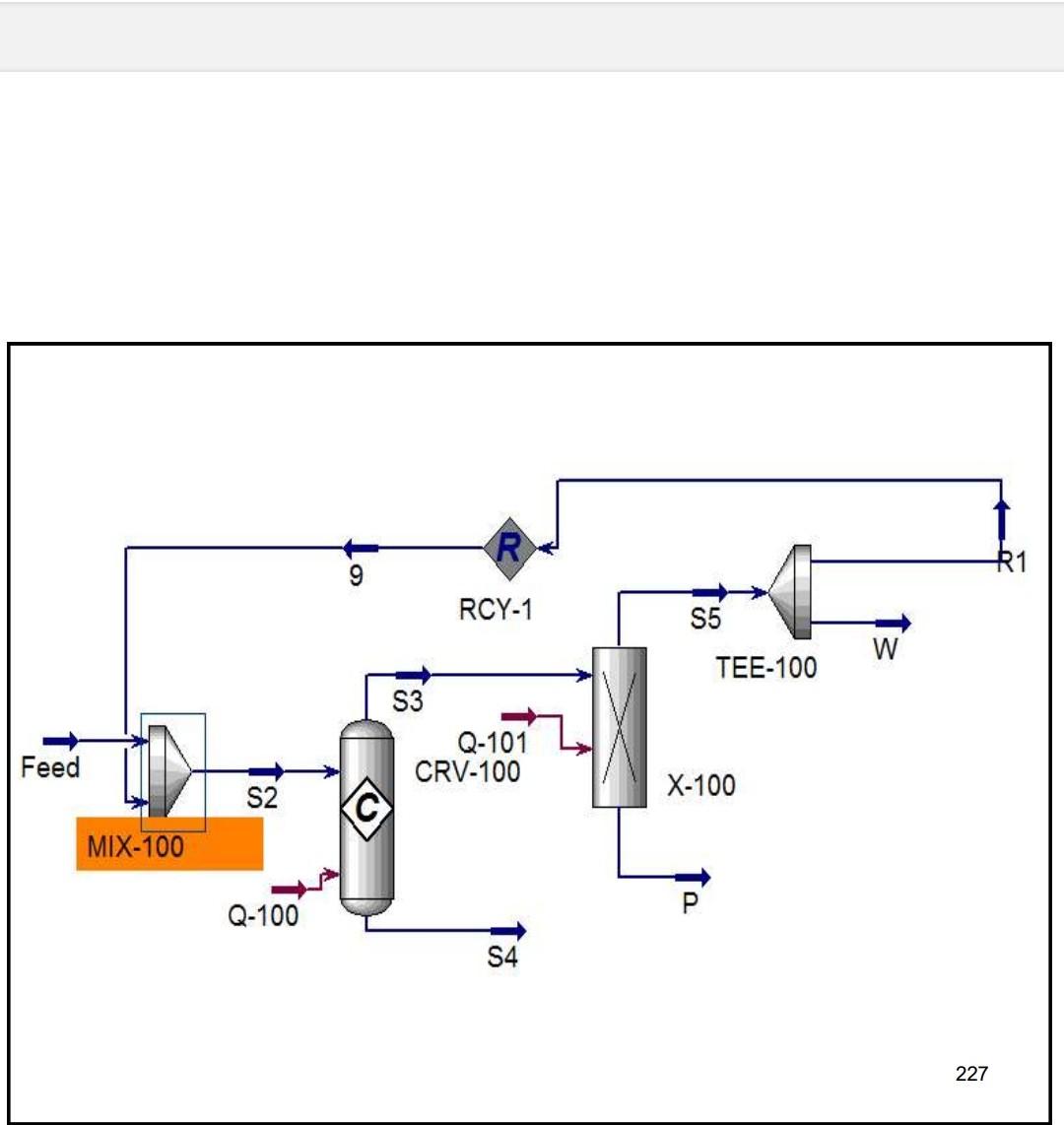
Example 4 In the process shown below, the feed stream is composed of 50% (mol) HCl, 48% (mol) C2H4 and 2% (mol) N2 at 100kgmol/hr,25C and 1 atm. Since the reaction separated from the unreacted reactants and the latter are recycled. The separation is performed in a distillation column, where a perfect separation is reached. The process is operated at the atmospheric pressure and pressure drops within the unit operations may be ignored. To prevent the accumulation of inerts in the process, 10kmol/hr is purged by stream IX Example 4 In the process shown below, the feed stream is composed of 50% (mol) HCl,48% (mol) C2H4 and 2% (mol) N2 at 100kgmol/hr,25C and 1 atm. Since the reaction C2H4+HClC2H5Cl achieves only 90mol% conversion, the ethylchloride product is separated from the unreacted reactants and the latter are recycled. The separation is performed in a distillation column, where a perfect separation is reached. The process is operated at the atmospheric pressure and pressure drops within the unit operations may be ignored. To prevent the accumulation of inerts in the process, 10kmol/hr is purged by stream W Completed. \begin{tabular}{|lr|} \hline Balance Error & 0.00000 \\ Reaction Heat (25C) & 7.2e+04k/kgmole \\ \hline \end{tabular} 221
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


