Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve any ONE from these two. step by step answer please only hand written accepted C05 Q5 Five moles of steam reacts with one mole
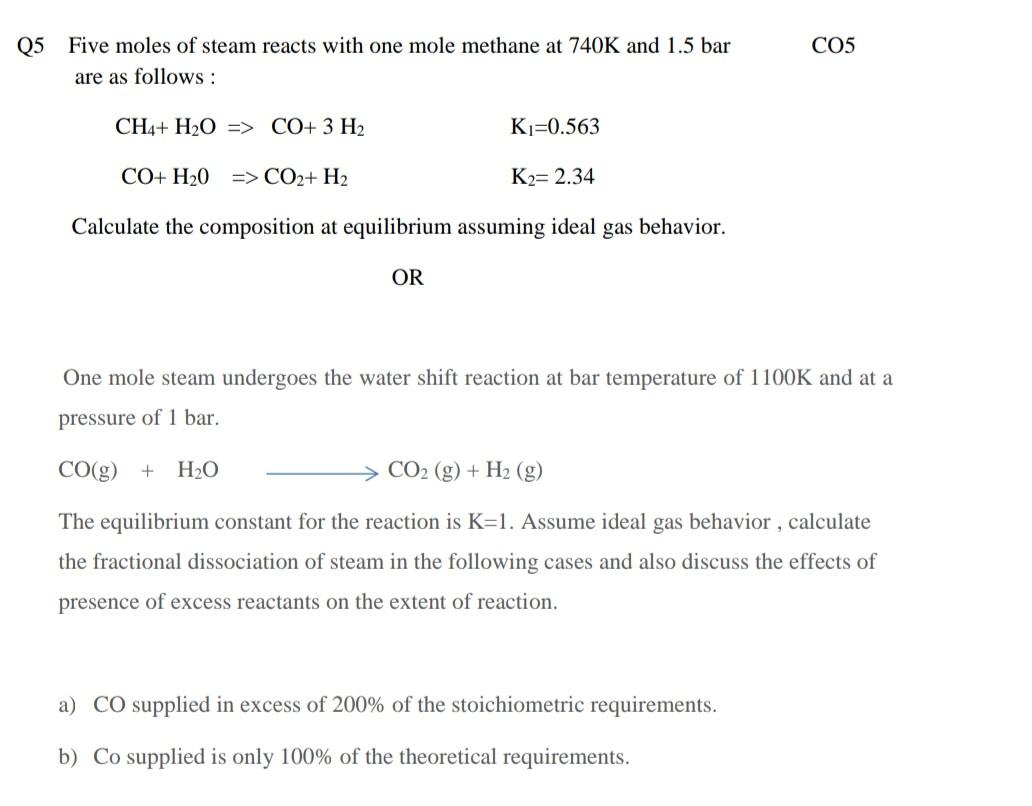
Solve any ONE from these two. step by step answer please only hand written accepted
C05 Q5 Five moles of steam reacts with one mole methane at 740K and 1.5 bar are as follows: CH4+ H2O => CO+ 3 H2 Ki=0.563 CO+ H2O => CO2+ H2 K2= 2.34 Calculate the composition at equilibrium assuming ideal gas behavior. OR One mole steam undergoes the water shift reaction at bar temperature of 1100K and at a pressure of 1 bar. CO(g) + H2O CO2 (g) + H2 (g) The equilibrium constant for the reaction is K=1. Assume ideal gas behavior, calculate the fractional dissociation of steam in the following cases and also discuss the effects of presence of excess reactants on the extent of reaction. a) CO supplied in excess of 200% of the stoichiometric requirements. b) Co supplied is only 100% of the theoretical requirementsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


