Question
Sometimes ortho and para isomers of methyl nitrobenzoate are formed in minor amounts, with the ortho isomer being preferred. Explain why the ortho isomer
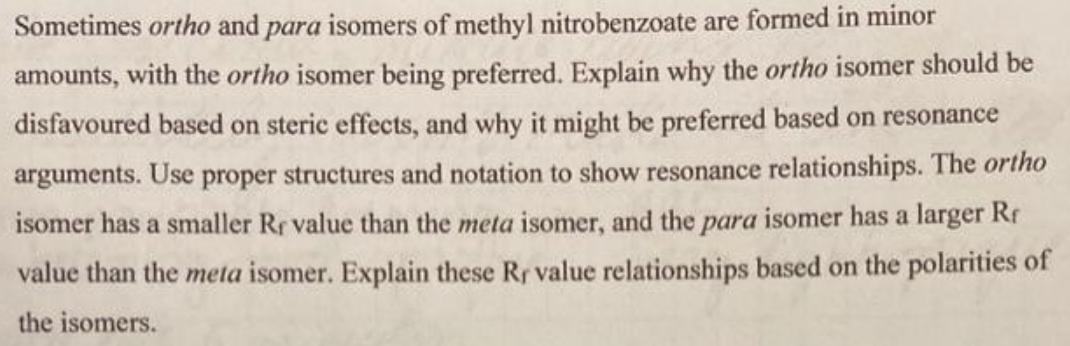
Sometimes ortho and para isomers of methyl nitrobenzoate are formed in minor amounts, with the ortho isomer being preferred. Explain why the ortho isomer should be disfavoured based on steric effects, and why it might be preferred based on resonance arguments. Use proper structures and notation to show resonance relationships. The ortho isomer has a smaller Rr value than the meta isomer, and the para isomer has a larger Rr value than the meta isomer. Explain these Rr value relationships based on the polarities of the isomers.
Step by Step Solution
3.57 Rating (185 Votes )
There are 3 Steps involved in it
Step: 1
Regulvin tor Nitratior ANO elechophile Come Claser to tengenu But otho carbon is Ang...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Microbiology A Systems Approach
Authors: Marjorie Kelly Cowan
4th edition
978-007340243, 73402435, 978-0073402437
Students also viewed these Biology questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



