Question
Task 1. 10 ml of an aqueous solution of phenol with a concentration of 10.0 mg/l was irradiated with a dose rate of 4.4
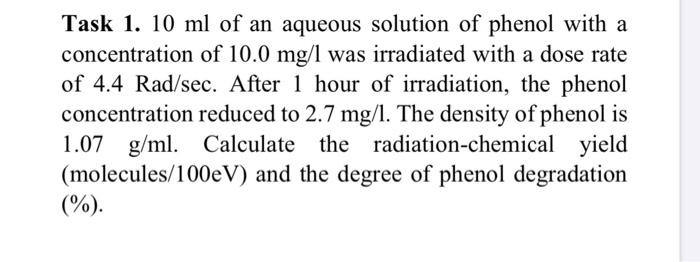
Task 1. 10 ml of an aqueous solution of phenol with a concentration of 10.0 mg/l was irradiated with a dose rate of 4.4 Rad/sec. After 1 hour of irradiation, the phenol concentration reduced to 2.7 mg/l. The density of phenol is 1.07 g/ml. Calculate the radiation-chemical yield (molecules/100eV) and the degree of phenol degradation (%).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Given that rate of radiation rate 44 radsec 2xpix f Therefore freq of incident li...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Separation process principles
Authors: J. D. Seader
2nd Edition
471464805, 978-0471464808
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



