Answered step by step
Verified Expert Solution
Question
1 Approved Answer
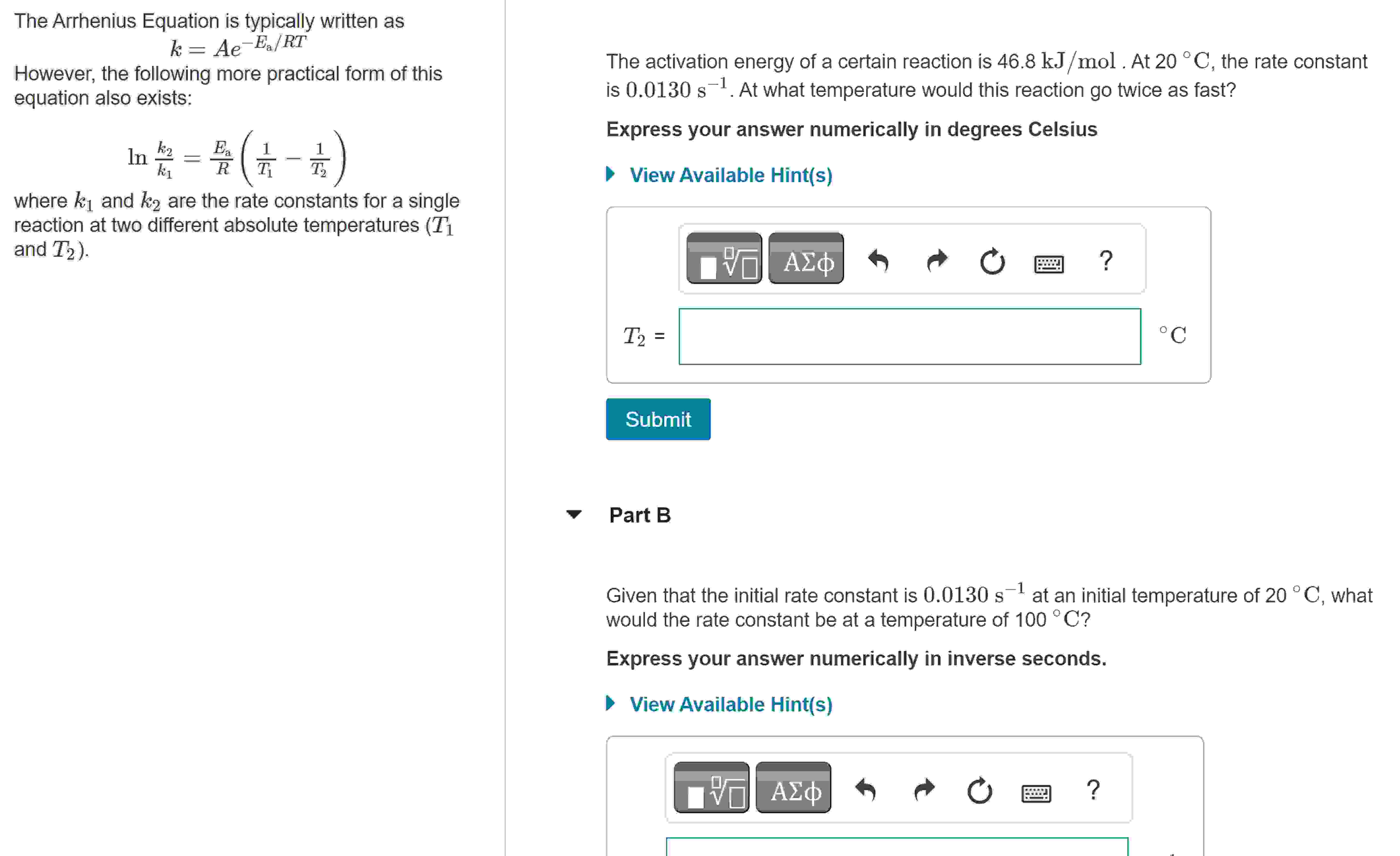
The Arrhenius Equation is typically written as k = A e - E a R T However, the following more practical form of this equation
The Arrhenius Equation is typically written as
However, the following more practical form of this
equation also exists:
where and are the rate constants for a single
reaction at two different absolute temperatures
and
The activation energy of a certain reaction is At the rate constant
is At what temperature would this reaction go twice as fast?
Express your answer numerically in degrees Celsius
View Available Hints
Part B
Given that the initial rate constant is at an initial temperature of what
would the rate constant be at a temperature of
Express your answer numerically in inverse seconds.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


