Question
A solution of an unknown nonvolatile NON ELECTROLYTE is prepared by adding 3.943 grams of the unknown to 70.61 grams of water. The boiling
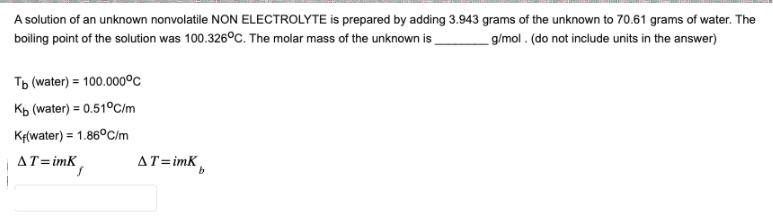
A solution of an unknown nonvolatile NON ELECTROLYTE is prepared by adding 3.943 grams of the unknown to 70.61 grams of water. The boiling point of the solution was 100.326C. The molar mass of the unknown is Tb (water) 100.000C Kb (water) = 0.51C/m K(water) 1.86C/m g/mol. (do not include units in the answer) AT=imK AT=imK b
Step by Step Solution
3.33 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION To solve this problem we can use the equation Tb Kb Kf x ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial and Managerial Accounting the basis for business decisions
Authors: Jan Williams, Susan Haka, Mark Bettner, Joseph Carcello
16th edition
0077664078, 978-0077664077, 78111048, 978-0078111044
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



