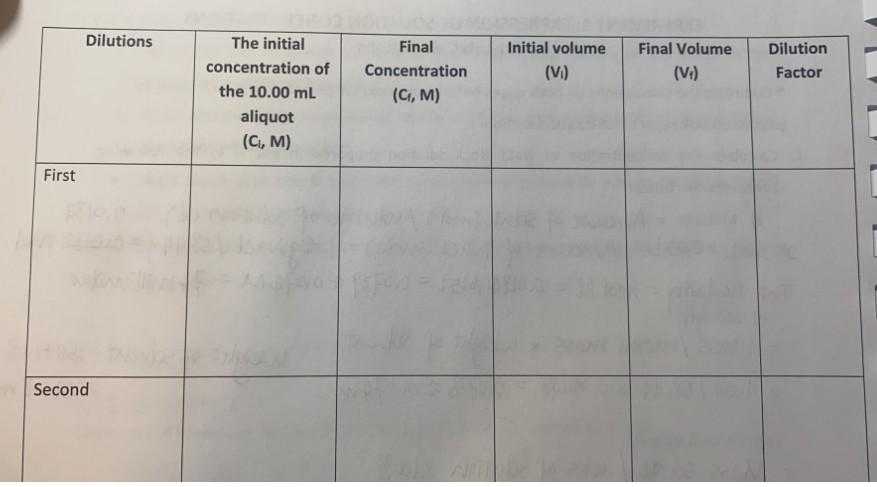
The experiment used 1.08g nacl. stock Nacl concentration 0.074M

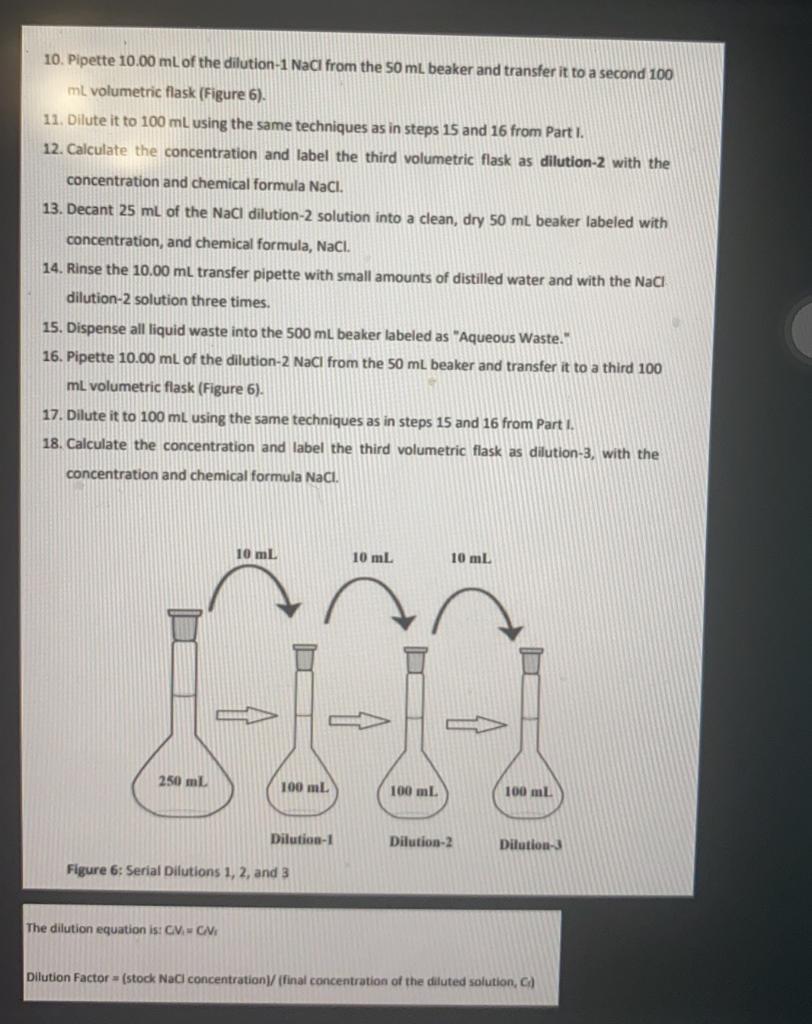

Part II: Serial Dilutions 1. Pour 25mL of the NaCl stock solution prepared in Part I into a clean, dry 50mL beaker (remember, NEVER pipette directly from a stock volumetric flask to prevent contamination of the stock solution). Label the 50mL beaker with the stock solution concentration and chemical formula, NaCl. 2. Next, rinse a 10.00mL transfer pipette three times with small amounts of distilled water and three times with the NaCl stock solution (Remember, the amount of liquid drawn should be just enough to start filling the broad, middle section of the pipette); refer to Figure 6. Figure 6: Rinsing the Volumetric Pipette. 3. Dispense all liquid waste into a 500mL beaker labeled "Aqueous Waste." 4. Pipette 10.00mL of the stock NaCl solution from the 50mL beaker and transfer it to a 100 mL volumetric flask. 5. Dilute it to 100mL using the same techniques as in steps 15 and 16 from Part 1 . 6. Calculate the concentration and label the 100mL volumetric flask as dilution-1, with NaCl concentration (M) and chemical formula. 7. Decant 25mL of the NaCl dilution-1 solution into a clean, dry 50mL beaker labeled with concentration, and chemical formula, NaCl. 8. Rinse the 10.00mL transfer pipette three times with small amounts of distilled water and three times with the NaCl dilution-1 solution (Figure 6). 9. Dispense all liquid waste from rinsing into the 500mL beaker labeled as "Aqueous Waste." 10. Pipette 10.00mL of the dilution-1 NaCi from the 50mL beaker and transfer it to a second 100 mL volumetric flask (Figure 6). 11. Dilute it to 100mL using the same techniques as in steps 15 and 16 from Part I. 12. Calculate the concentration and label the third volumetric flask as dilution-2 with the concentration and chemical formula NaCl. 13. Decant 25mL of the NaCl dilution-2 solution into a clean, dry 50mL beaker labeled with concentration, and chemical formula, NaCl. 14. Rinse the 10.00mL transfer pipette with small amounts of distilled water and with the NaCl dilution-2 solution three times. 15. Dispense all liquid waste into the 500mL beaker labeled as "Aqueous Waste." 16. Pipette 10.00mL of the dilution-2 NaCl from the 50mL beaker and transfer it to a third 100 mL volumetric flask (Figure 6). 17. Dilute it to 100mL using the same techniques as in steps 15 and 16 from Part I. 18. Calculate the concentration and label the third volumetric flask as dilution-3, with the concentration and chemical formula NaCi. Figure 6: Senal pilutions 1, 2, and 3 The dilution equation is CV1=CNi Dilution Factor = (stock NaC concentration)/ (final concentration of the dilluted solution, C) Stock NaCl Concentration 0,074 . M (prepared in Part I) The dilution equation is: CiVi=CfVf Dilution Factor =( stock NaCl concentration )/ (final concentration of the diluted solution, Cf)










