Question
The figure below provides the observed concentration of SF6 (ppm) at recording stations. According to the US EPA, SF6 is the most potent greenhouse gas
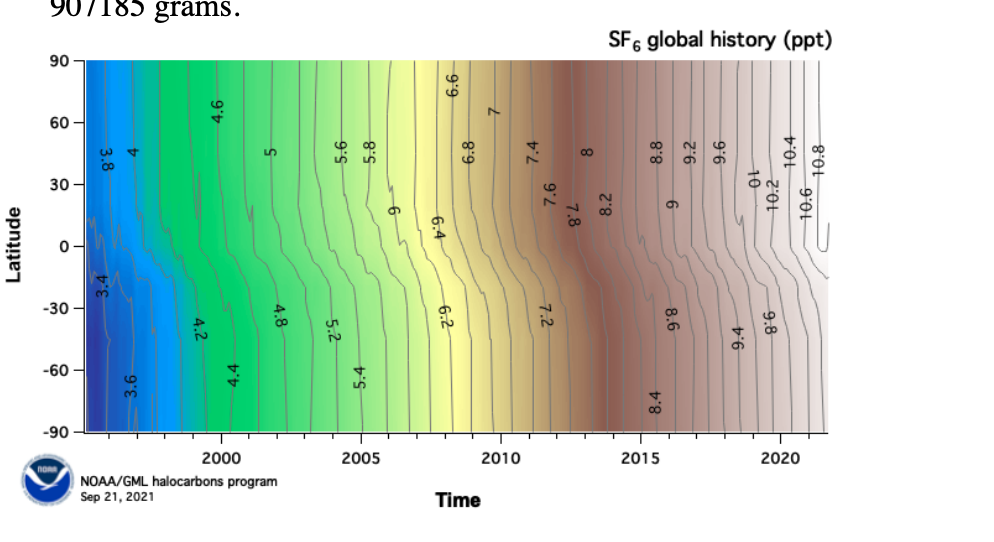
The figure below provides the observed concentration of SF6 (ppm) at recording stations. According to the US EPA, "SF6 is the most potent greenhouse gas known. It is 22,800 times more effective at trapping infrared radiation than an equivalent amount of CO2 and stays in the atmosphere for 3,200 years."
a. Calculate how many kilotons of SF6 was released in the year 2002. The atmosphere contains 1.8 x 1020 moles of gases, 1 ton = 907185 grams.
b. If the rate of SF6 emissions remains the same, how much SF6 (ppm) will be in the environment when you are 50? If SF6 emissions are completely halted, how much SF6 (ppm) will be in the environment when you are 50?
2. The water in the Flint water system in mandated to have a phosphate concentrations above 3.1 ppm and a pH above 7.0. a. Assuming that the phosphate concentration [PO4 3- ] is 3.1 ppm and the pH is 7.46, what is the concentration of Pb2+ (Ksp = 3.010-44)? According to Environ. Sci. Technol. 1978, 12, 13, 1406-1410. The lead hydroxide species, Pb(OH)+, Pb(OH)2, and Pb(OH)3 - have the following respective b values; b1 = 6.57, log b2 = 10.80, log b3 = 13.63
b. Write balanced cumulative formation equations for each lead hydroxide species.
c. Calculate the concentration of each lead hydroxide species, assuming the pH is maintained at 7.46 and the phosphate concentration [PO4 3- ] remains 3.1 ppm.
d. Calculate the total concentration of all lead species in the water. Is it above or below the US EPA limit of 15 ppb? Explain your answer.
907185 grams. SF global history (ppt) 6 06 6.6 60 5.6 5.8 6.8 7.4 8 8.8 26 9.6 10 30 - 10.4 10.8 10.2 10.6 7.6 8.2 9 7.8 Latitude 64 -30 - 4.8 6.2 7.2 4.2 5.2 8.6 9.8 9.4 -60- 4.4 5.4 -90 2005 2010 2015 2020 2000 NOAA/GML halocarbons program Sep 21, 2021 Time 907185 grams. SF global history (ppt) 6 06 6.6 60 5.6 5.8 6.8 7.4 8 8.8 26 9.6 10 30 - 10.4 10.8 10.2 10.6 7.6 8.2 9 7.8 Latitude 64 -30 - 4.8 6.2 7.2 4.2 5.2 8.6 9.8 9.4 -60- 4.4 5.4 -90 2005 2010 2015 2020 2000 NOAA/GML halocarbons program Sep 21, 2021 TimeStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


