Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The 'molecular inversion of the (Cp)2Be, in which exchange between 1 and 5 coordination modes can be shown only in the solid state. Select one:
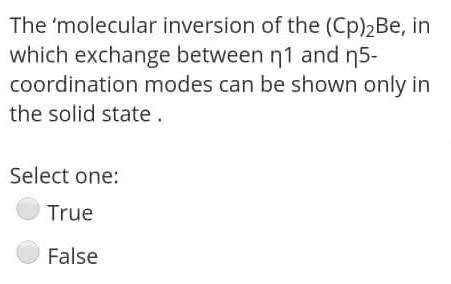


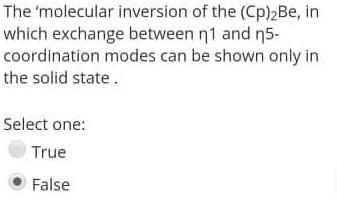



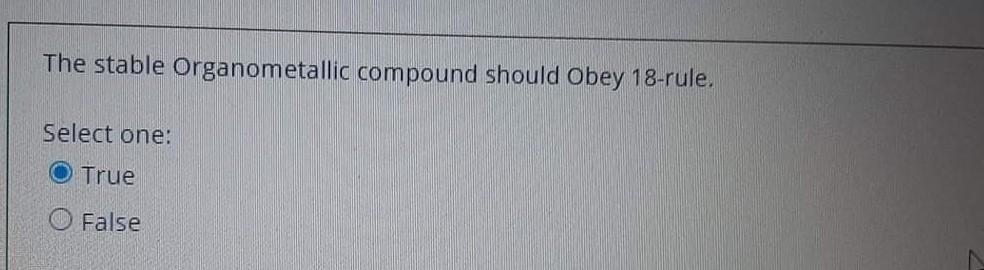





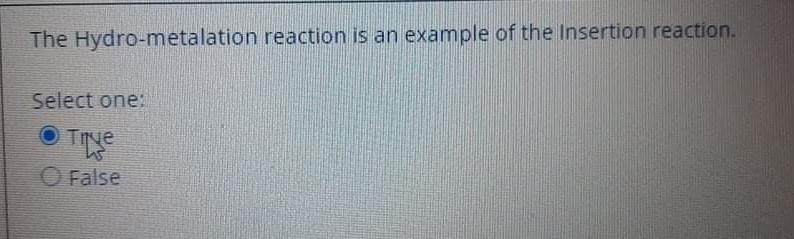








The 'molecular inversion of the (Cp)2Be, in which exchange between 1 and 5 coordination modes can be shown only in the solid state. Select one: True False The stable Organometallic compound should Obey 18-rule. Select one: True False Organometallic compound don't have a covalent bond. Select one: True False The 'molecular inversion of the (Cp)2Be, in which exchange between 1 and 5 coordination modes can be shown only in the solid state. Select one: True False Decarboxylation of organelement-formats leads to 1 alcohols. Select one: True False Carboxylates should contain electron-donating substituents to undergoes an elimination reaction. Select one: True False Organometallic compound don't have a covalent bond. Select one: True False The stable Organometallic compound should Obey 18-rule. Select one: True False The utility of Organometallic's is to make a nucleophilic carbon attack an electrophilic carbon to perform C-C bond. Select one: True False Carboxylates should contain electron-donating substituents to undergoes an elimination reaction. Select one: True False The key concepts of Organometallic is to make a carbon behave as electrophilic species. Select one: True False The utility of Organometallic's is to make a nucleophilic carbon attack an electrophilic carbon to perform C-C bond. Select one: True False The reaction ( RM+RXRM+RX), considered as Competing reaction. Select one: True False The Hydro-metalation reaction is an example of the insertion reaction. Select one: False The fluxional process Be-Cyclopentadiene compound makes all the proton and all the carbon environments equivalent during the NMR-spectroscopy. Select one: True False The most common use of RMgX or RLi is to from a new carbon - metal bonds. Select one: True False The 'molecular inversion of the (Cp)2Be, in which exchange between 1 and 5-coordination modes can be shown only in the solid state. Select one: True False The fluxional process Be-cyclopentadiene compound makes all the proton and all the carbon environments equivalent during the NMR-spectroscopy. Select one: True False Carboxylates should contain electrondonating substituents to undergoes an elimination reaction. Select one: True False The utility of organometallic compounds is their usage as a source of nucleophilic Carbon. Select one: True False The pyrophoric material of alkali metal organometallics can be stabilizing by complexing with 1,2-dimethoxyethane (dme). Select one: True Decarboxylation of organelement-formats leads to 1 alcohols. Select one: True False The 'molecular inversion of the (Cp)2Be, in which exchange between 1 and 5 coordination modes can be shown only in the solid state. Select one: True False The stable Organometallic compound should Obey 18-rule. Select one: True False Organometallic compound don't have a covalent bond. Select one: True False The 'molecular inversion of the (Cp)2Be, in which exchange between 1 and 5 coordination modes can be shown only in the solid state. Select one: True False Decarboxylation of organelement-formats leads to 1 alcohols. Select one: True False Carboxylates should contain electron-donating substituents to undergoes an elimination reaction. Select one: True False Organometallic compound don't have a covalent bond. Select one: True False The stable Organometallic compound should Obey 18-rule. Select one: True False The utility of Organometallic's is to make a nucleophilic carbon attack an electrophilic carbon to perform C-C bond. Select one: True False Carboxylates should contain electron-donating substituents to undergoes an elimination reaction. Select one: True False The key concepts of Organometallic is to make a carbon behave as electrophilic species. Select one: True False The utility of Organometallic's is to make a nucleophilic carbon attack an electrophilic carbon to perform C-C bond. Select one: True False The reaction ( RM+RXRM+RX), considered as Competing reaction. Select one: True False The Hydro-metalation reaction is an example of the insertion reaction. Select one: False The fluxional process Be-Cyclopentadiene compound makes all the proton and all the carbon environments equivalent during the NMR-spectroscopy. Select one: True False The most common use of RMgX or RLi is to from a new carbon - metal bonds. Select one: True False The 'molecular inversion of the (Cp)2Be, in which exchange between 1 and 5-coordination modes can be shown only in the solid state. Select one: True False The fluxional process Be-cyclopentadiene compound makes all the proton and all the carbon environments equivalent during the NMR-spectroscopy. Select one: True False Carboxylates should contain electrondonating substituents to undergoes an elimination reaction. Select one: True False The utility of organometallic compounds is their usage as a source of nucleophilic Carbon. Select one: True False The pyrophoric material of alkali metal organometallics can be stabilizing by complexing with 1,2-dimethoxyethane (dme). Select one: True Decarboxylation of organelement-formats leads to 1 alcohols. Select one: True False
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


