Answered step by step
Verified Expert Solution
Question
1 Approved Answer
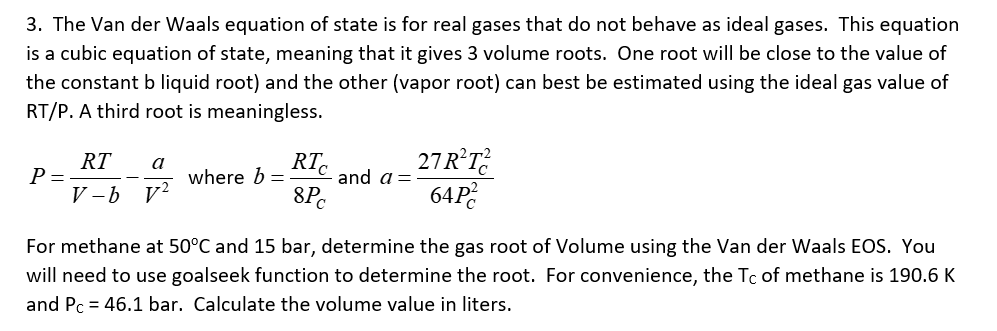
The Van der Waals equation of state is for real gases that do not behave as ideal gases. This equation is a cubic equation of
The Van der Waals equation of state is for real gases that do not behave as ideal gases. This equation
is a cubic equation of state, meaning that it gives volume roots. One root will be close to the value of
the constant liquid root and the other vapor root can best be estimated using the ideal gas value of
RTP A third root is meaningless.
where and
For methane at and bar, determine the gas root of Volume using the Van der Waals EOS. You
will need to use goalseek function to determine the root. For convenience, the of methane is
and bar. Calculate the volume value in liters.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


