Answered step by step
Verified Expert Solution
Question
1 Approved Answer
- The volumetric flow rate of the gas feed: 40m3/hr - The concentration of C2H6 in the feed stream: 5kmol/m3 - Reaction rate is first


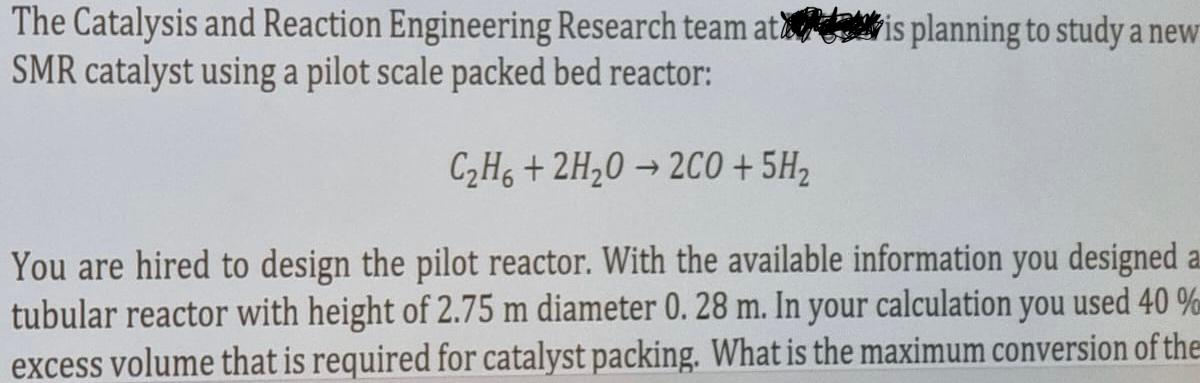 - The volumetric flow rate of the gas feed: 40m3/hr - The concentration of C2H6 in the feed stream: 5kmol/m3 - Reaction rate is first order in terms of oxygen: r=2CC2H6(kmol/hr.Kg) - Density of the catalyst particle: 1000kg/m3 - Bed porosity, :0.5 - The design equation for the packed bed reactor: 0WdW=kcA0FA00XA1XAdXA The Catalysis and Reaction Engineering Research team at SMR catalyst using a pilot scale packed bed reactor: C2H6+2H2O2CO+5H2 You are hired to design the pilot reactor. With the available information you designe tubular reactor with height of 2.75m diameter 0.28m. In your calculation you used 4 excess volume that is required for catalyst packing. What is the maximum conversion of limiting reactant C2H6 you can achieve in this reactor? The following informatio available for your calculation. The Catalysis and Reaction Engineering Research team at is planning to study a new SMR catalyst using a pilot scale packed bed reactor: C2H6+2H2O2CO+5H2 You are hired to design the pilot reactor. With the available information you designed tubular reactor with height of 2.75m diameter 0.28m. In your calculation you used 40% excess volume that is required for catalyst packing. What is the maximum conversion of the
- The volumetric flow rate of the gas feed: 40m3/hr - The concentration of C2H6 in the feed stream: 5kmol/m3 - Reaction rate is first order in terms of oxygen: r=2CC2H6(kmol/hr.Kg) - Density of the catalyst particle: 1000kg/m3 - Bed porosity, :0.5 - The design equation for the packed bed reactor: 0WdW=kcA0FA00XA1XAdXA The Catalysis and Reaction Engineering Research team at SMR catalyst using a pilot scale packed bed reactor: C2H6+2H2O2CO+5H2 You are hired to design the pilot reactor. With the available information you designe tubular reactor with height of 2.75m diameter 0.28m. In your calculation you used 4 excess volume that is required for catalyst packing. What is the maximum conversion of limiting reactant C2H6 you can achieve in this reactor? The following informatio available for your calculation. The Catalysis and Reaction Engineering Research team at is planning to study a new SMR catalyst using a pilot scale packed bed reactor: C2H6+2H2O2CO+5H2 You are hired to design the pilot reactor. With the available information you designed tubular reactor with height of 2.75m diameter 0.28m. In your calculation you used 40% excess volume that is required for catalyst packing. What is the maximum conversion of the Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


