Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thermodynamics. pls provide full answers with legible handwriting, thanks. You have been asked to prepare a 2000cm3 liquid solution consisting of 30mol% propane (1) and

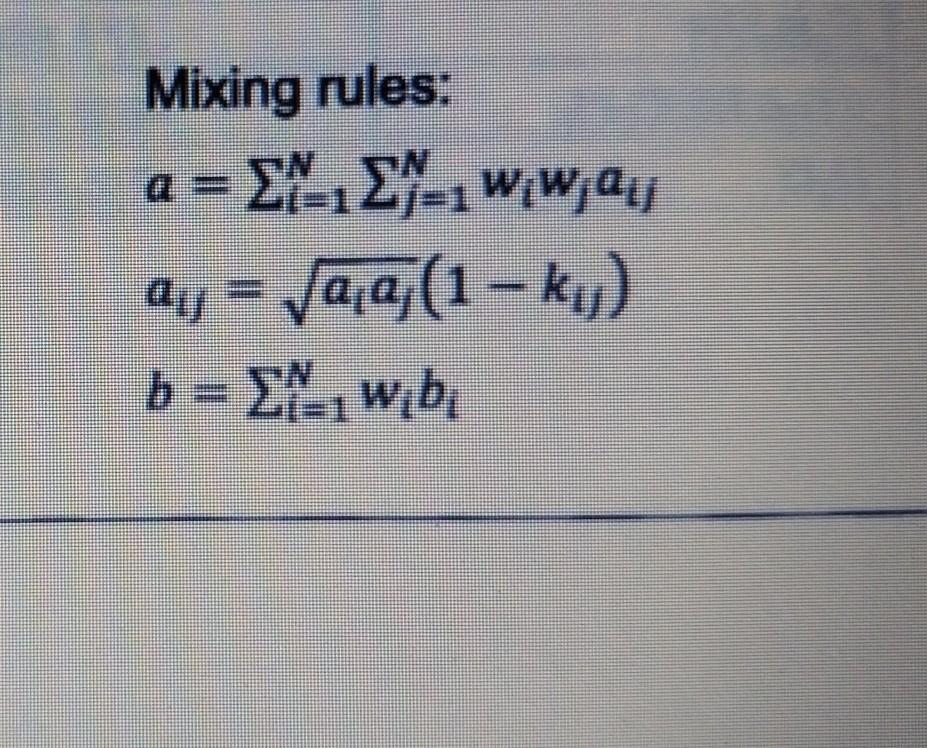
thermodynamics. pls provide full answers with legible handwriting, thanks.
You have been asked to prepare a 2000cm3 liquid solution consisting of 30mol% propane (1) and 70mol% n-pentane (2) at 305K and 1atm. The calculated thermodynamic properties using Peng-Robinson equation of state are shown in Table 1. a) Determine the compressibility factor and the molar volume of the solution, given that a12=17,514,536cm6 bar /mol2 and ki=0.00098. (7 marks) b) Calculate the partial molar volume for n-pentane. (2 marks) c) Determine the individual volumes of propane and n-pentane that are required to be poured in to obtain a 2000cm3 solution. (4 marks) d) Is the intermolecular attractive force dominant in this solution? (2 marks) Jadual 1: Sifat-sifat termodinamik propana (1) dan n-pentana (2) pada 305K dan 1atm Table 1: Thermodynamic properties of propane (1) and n-pentane (2) at 305K and 1atm Mixing rules: a=i=1Nj=1Nwiwjaijaij=aiaj(1kij)b=i=1Nwibi You have been asked to prepare a 2000cm3 liquid solution consisting of 30mol% propane (1) and 70mol% n-pentane (2) at 305K and 1atm. The calculated thermodynamic properties using Peng-Robinson equation of state are shown in Table 1. a) Determine the compressibility factor and the molar volume of the solution, given that a12=17,514,536cm6 bar /mol2 and ki=0.00098. (7 marks) b) Calculate the partial molar volume for n-pentane. (2 marks) c) Determine the individual volumes of propane and n-pentane that are required to be poured in to obtain a 2000cm3 solution. (4 marks) d) Is the intermolecular attractive force dominant in this solution? (2 marks) Jadual 1: Sifat-sifat termodinamik propana (1) dan n-pentana (2) pada 305K dan 1atm Table 1: Thermodynamic properties of propane (1) and n-pentane (2) at 305K and 1atm Mixing rules: a=i=1Nj=1Nwiwjaijaij=aiaj(1kij)b=i=1NwibiStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


