Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This question is attached with 2 pictures but of the same question. Like on this question, devide your answer to 5 and add short explanation

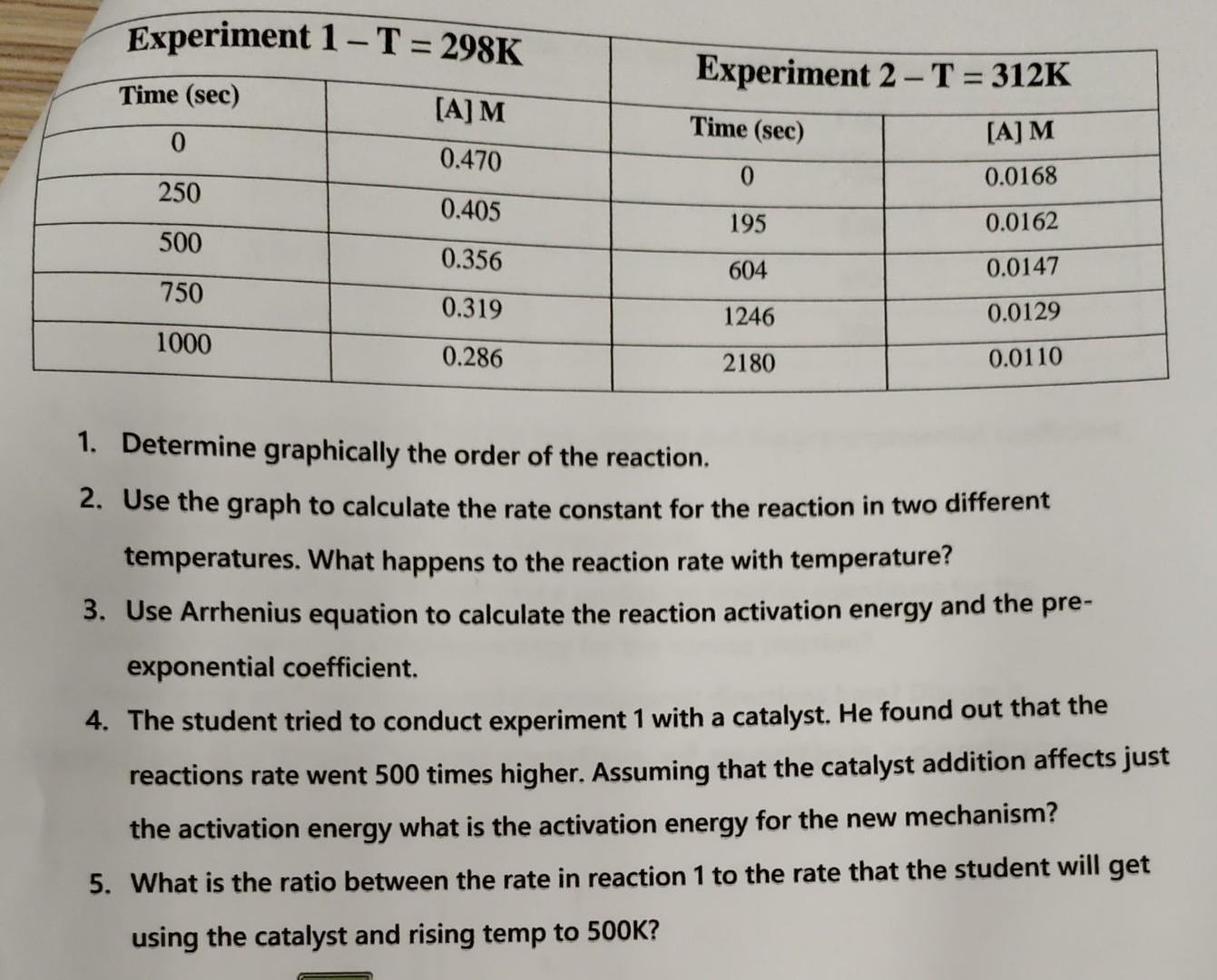
This question is attached with 2 pictures but of the same question. Like on this question, devide your answer to 5 and add short explanation that I can undsrstand.
dependency The elementary reaction AB+2C was investigated under two different reaction temperatures. The results are in the following table: 1. Determine graphically the order of the reaction. 2. Use the graph to calculate the rate constant for the reaction in two different temperatures. What happens to the reaction rate with temperature? 3. Use Arrhenius equation to calculate the reaction activation energy and the preexponential coefficient. 4. The student tried to conduct experiment 1 with a catalyst. He found out that the reactions rate went 500 times higher. Assuming that the catalyst addition affects just the activation energy what is the activation energy for the new mechanism? 5. What is the ratio between the rate in reaction 1 to the rate that the student will get using the catalyst and rising temp to 500KStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


