Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Consider the following temperature-composition diagram of a high-boiling azeotrope. a,a, a, a-ba, Vapour composition Boiling temperature of liquid b a Mole fraction of
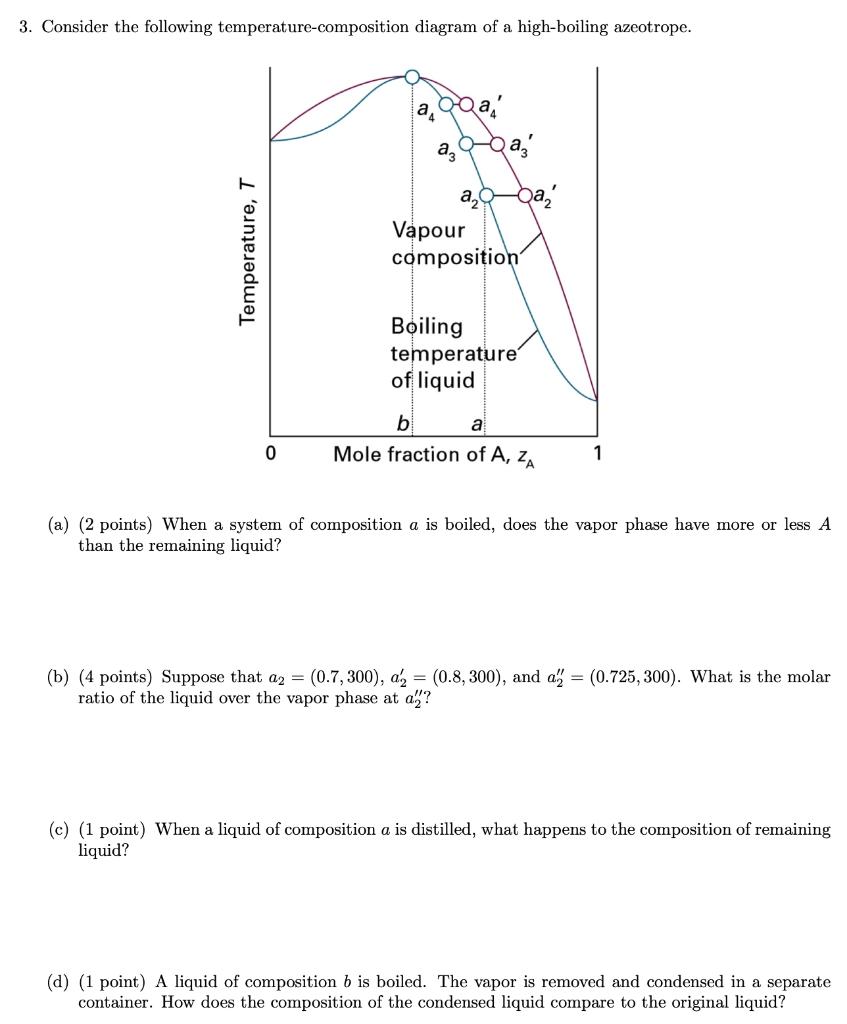
3. Consider the following temperature-composition diagram of a high-boiling azeotrope. a,a, a, a-ba, Vapour composition Boiling temperature of liquid b a Mole fraction of A, z, 1 (a) (2 points) When a system of composition a is boiled, does the vapor phase have more or less A than the remaining liquid? (b) (4 points) Suppose that az = (0.7, 300), a, = (0.8, 300), and a" = (0.725, 300). What is the molar ratio of the liquid over the vapor phase at a? (c) (1 point) When a liquid of composition a is distilled, what happens to the composition of remaining liquid? (d) (1 point) A liquid of composition b is boiled. The vapor is removed and condensed in a separate container. How does the composition of the condensed liquid compare to the original liquid? Temperature, T
Step by Step Solution
★★★★★
3.30 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
vafos Couperilice a Mole Frachua with composiheu q the vap...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


