Question
We've dealt with the molecular diagram of diatomic molecules. However, conceptually the problem is much more difficult when dealing multi-atomic molecules. Below is shown
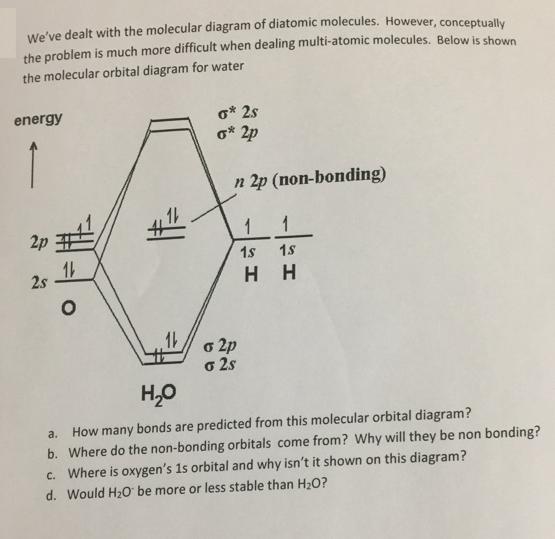
We've dealt with the molecular diagram of diatomic molecules. However, conceptually the problem is much more difficult when dealing multi-atomic molecules. Below is shown the molecular orbital diagram for water o* 2s o* 2p energy n 2p (non-bonding) 1 1 2p 1s 18 2s H H o 2p o 2s H,0 How many bonds are predicted from this molecular orbital diagram? b. Where do the non-bonding orbitals come from? Why will they be non bonding? c. Where is oxygen's 1s orbital and why isn't it shown on this diagram? a. d. Would H2o be more or less stable than H2O?
Step by Step Solution
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
a four bonds are predicted from this molecular orbital diag...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



