Answered step by step
Verified Expert Solution
Question
1 Approved Answer
what is the change in specific enthalpy from saturated liquid at 20 bar to saturated vapor at 20 bar? ive tried 980 and a little
what is the change in specific enthalpy from saturated liquid at 20 bar to saturated vapor at 20 bar? ive tried 980 and a little over 900 KJ/Kg and im still getting the problem incorrect. (bonus: can you calculate the heating without phase change as well?) 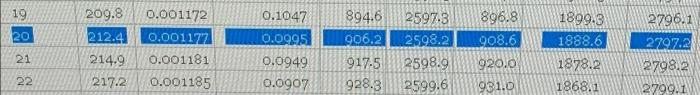
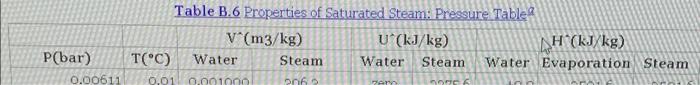
0.1047 894.6 19 20 0.001172 0.001177 0.001181 0.0995 209.8 212.4 214.9 217.2 906.2 2597.3 2598.2 2598.9 2599.6 896.8 908.6 920.0 1899.3 1888.6 1878.2 1868.1 21 2796.1 27972 2798.2 2799.1 0.0949 0.0907 917-5 928.3 22 0.001185 981.0 Table B.6 Properties of Saturated Steam: Pressure Tabler V(m3/kg) U (kJ/kg) AH (kJ/kg) T(C) Water Steam Water Steam Water Evaporation Steam P(bar) 0.00611 0.01 0.001000 206 Per 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


