Question
A solution contains 0.356 M NH4Br and 7.8210-2 M ammonia. The pH of this solution is Write the net ionic equation for the equilibrium
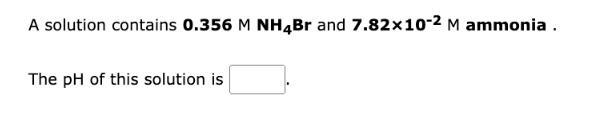

A solution contains 0.356 M NH4Br and 7.8210-2 M ammonia. The pH of this solution is Write the net ionic equation for the equilibrium that is established when barium hypochlorite is dissolved in water. It is not necessary to include states such as (aq) or (!). This solution is + HO = +
Step by Step Solution
3.51 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Understanding Cross Cultural Management
Authors: Marie Joelle Browaeys, Roger Price
3rd Edition
1292015896, 978-1292015897
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



