Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Which chemical equation is an example of a neutralization reaction? 2KCIO3 2KCI+ 30 OHCI + NaOH NaCl + HO OCH4 +202 2CO2 + 2HO

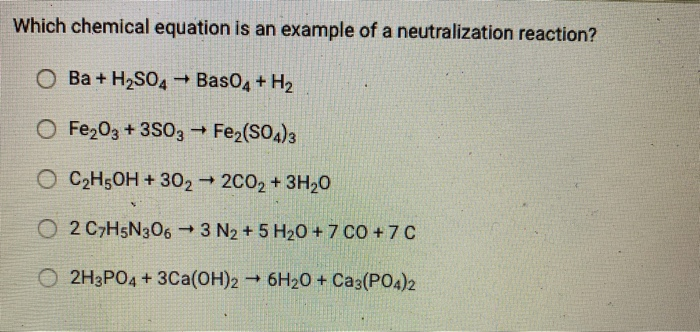
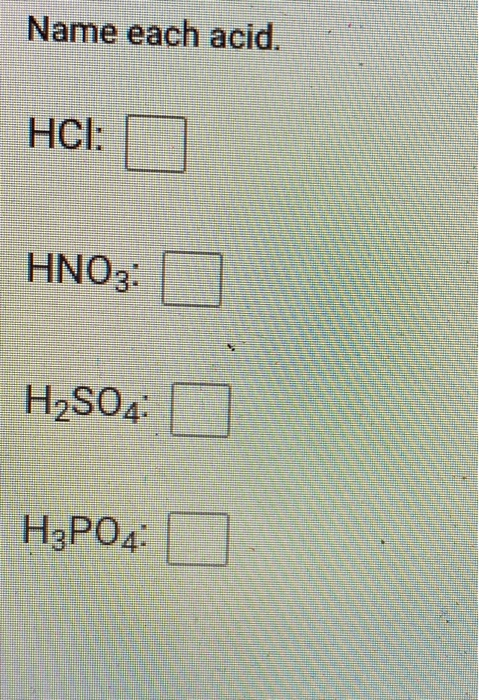
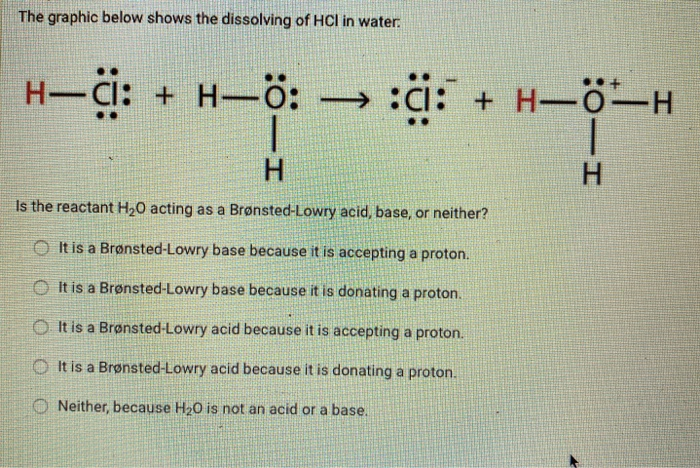
Which chemical equation is an example of a neutralization reaction? 2KCIO3 2KCI+ 30 OHCI + NaOH NaCl + HO OCH4 +202 2CO2 + 2HO 2Na+ Cl 2NaCl Zn+ 2HCl ZnCl + H Which chemical equation is an example of a neutralization reaction? O Ba + HSO4 BasO4 + H O FeO3 + 3SO3 ->> Fe(SO4)3 OCH5OH + 302 2CO2 + 3HO O2 C7H5N3063 N + 5 H0 + 7 C0 +7C O2H3PO4 + 3Ca(OH)2 6HO + Ca3(PO4)2 Name each acid. HCI: HNO3: HSO4: HPO4 The graphic below shows the dissolving of HCI in water. + H: :ci: 1 H Is the reactant H0 acting as a Brnsted-Lowry acid, base, or neither? It is a Brnsted-Lowry base because it is accepting a proton. It is a Brnsted-Lowry base because it is donating a proton. It is a Brnsted-Lowry acid because it is accepting a proton. It is a Brnsted-Lowry acid because it is donating a proton. Neither, because HO is not an acid or a base. H-CI: + :01H + HH
Step by Step Solution
★★★★★
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below 1 Neutralization rxn Acid Base Salt Wat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


