Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Work a problem similar to P 3 . 7 . 6 in the textbook. Differences: a . Find the degrees of freedom. b . What
Work a problem similar to P in the textbook. Differences:
a Find the degrees of freedom.
b What assumptions are inherent in the modeling?
c Calculate the final steadystate heights before you do any modeling. You can use these to check your Python code.
d Use Python instead of Matlab to solve the problem. Submit your graph for the heights as a function of time.
e Repeat part d with a decrease in Fspec. Submit your graph for the heights as a function of time.
f Submit your Python file.
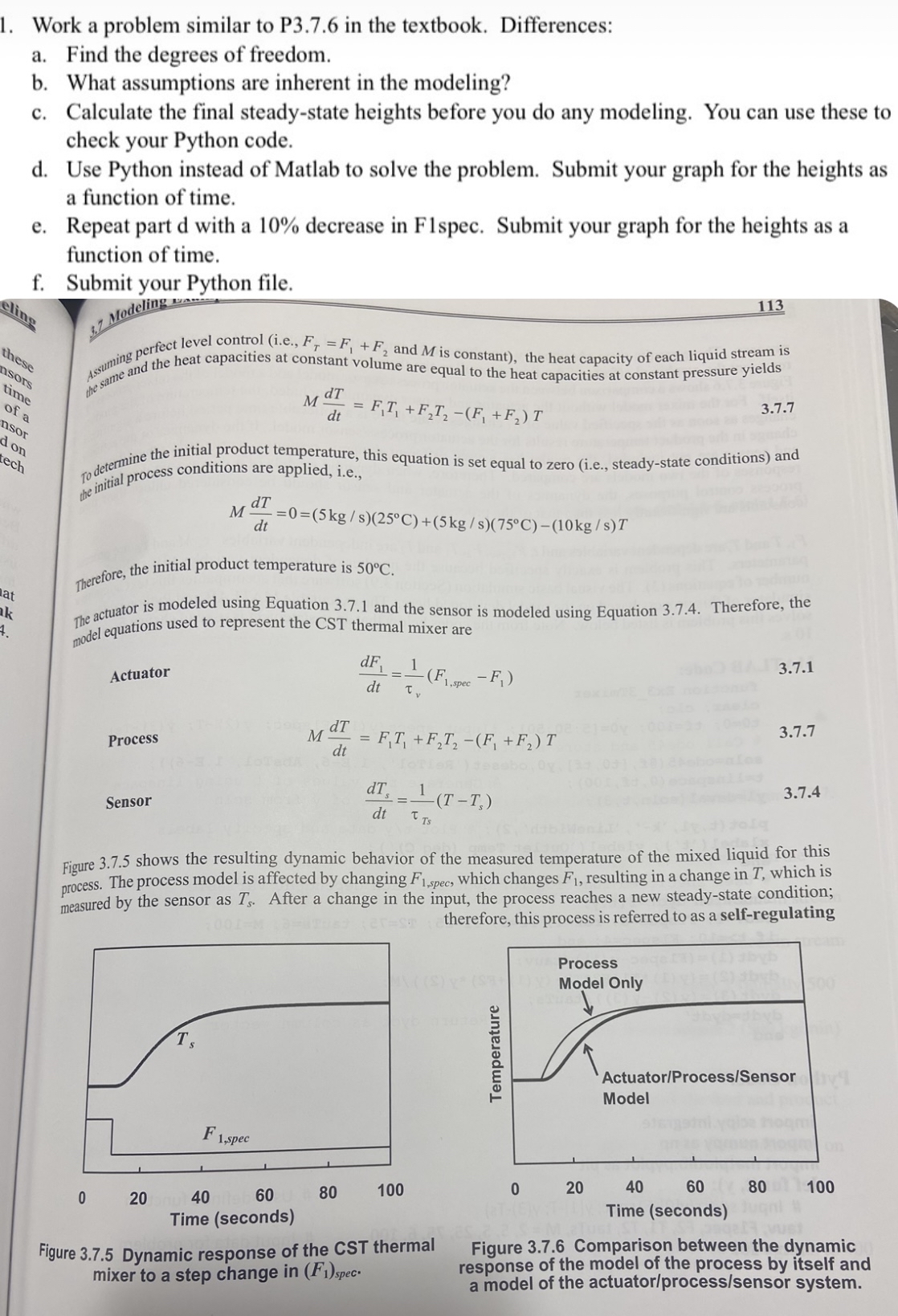
To determine the initial product temperature, this equation is set equal to zero ie steadystate conditions and the initial process conditions are applied, ie
Therefore, the initial product temperature is
The actuator is modeled using Equation and the sensor is modeled using Equation Therefore, the model equations used to represent the CST thermal mixer are
Actuator
Process
Sensor
Figure shows the resulting dynamic behavior of the measured temperature of the mixed liquid for this process. The process model is affected by changing which changes resulting in a change in which is measured by the sensor as After a change in the input, the process reaches a new steadystate condition; therefore, this process is referred to as a selfregulating
Figure Dynamic response of the CST thermal mixer to a step change in
Figure Comparison between the dynamic response of the model of the process by itself and a model of the actuatorprocesssensor system.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


