Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You can refer to these tables for values. Please give full solution with steps. Will upvote 4. (13 marks total) The sketch shows a simple
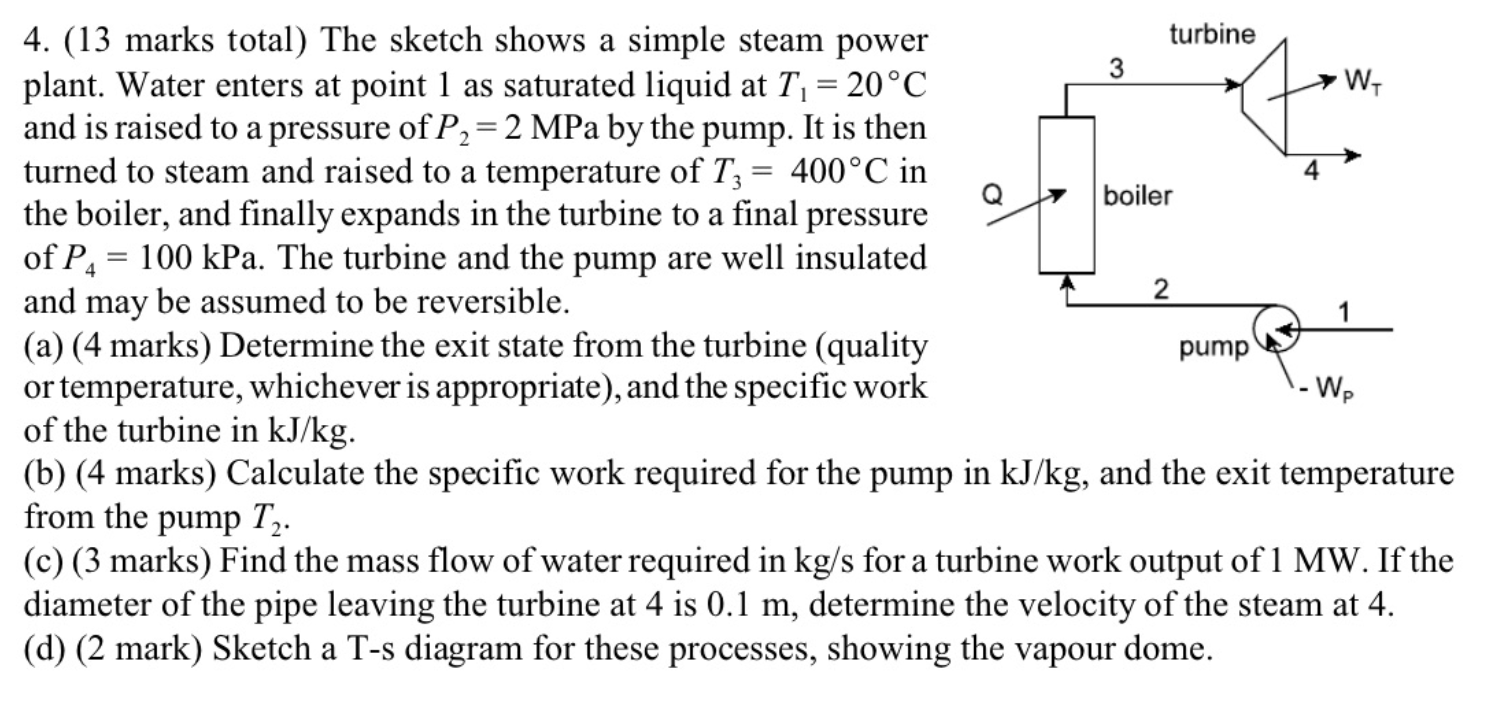
You can refer to these tables for values. Please give full solution with steps. Will upvote
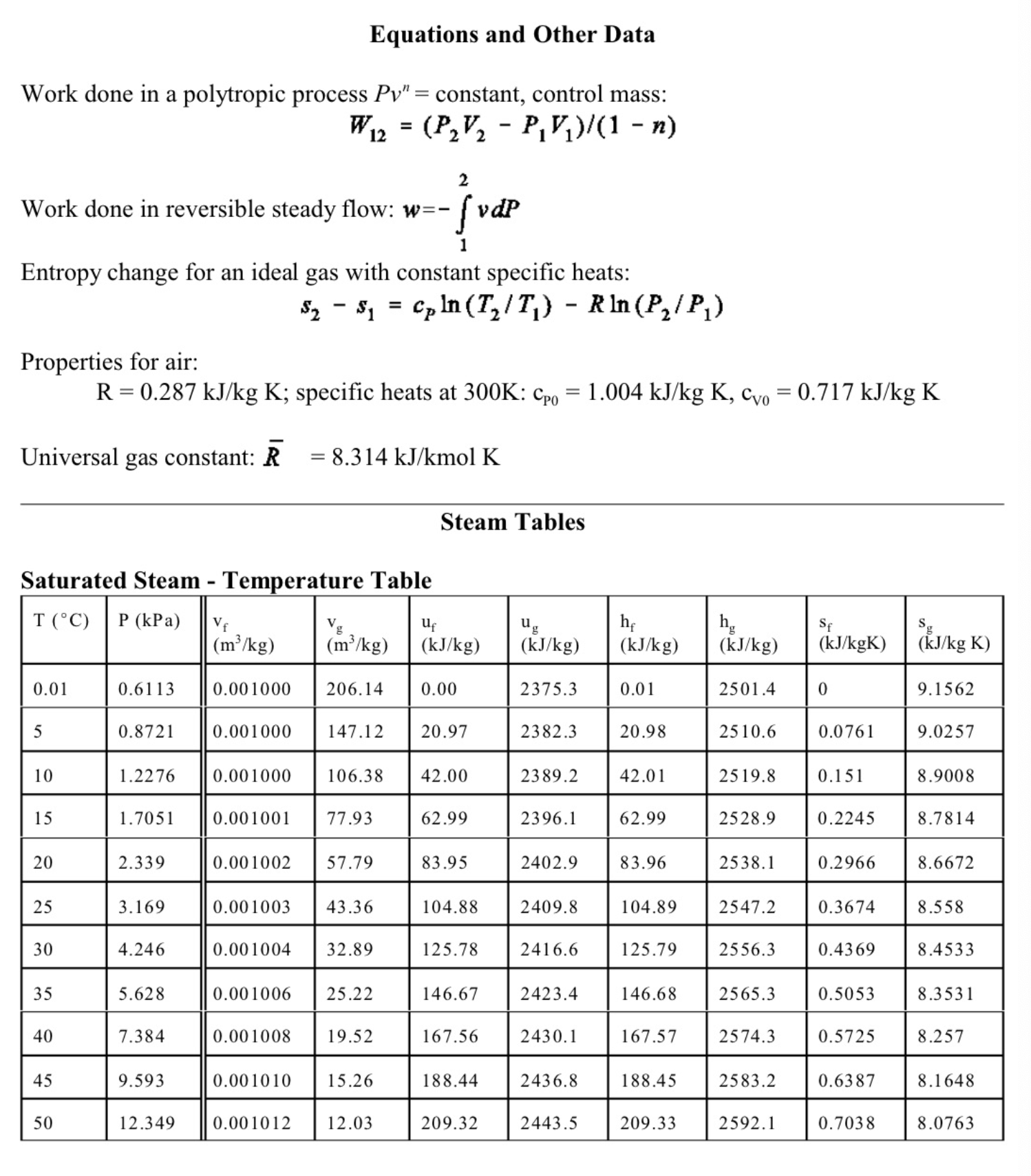
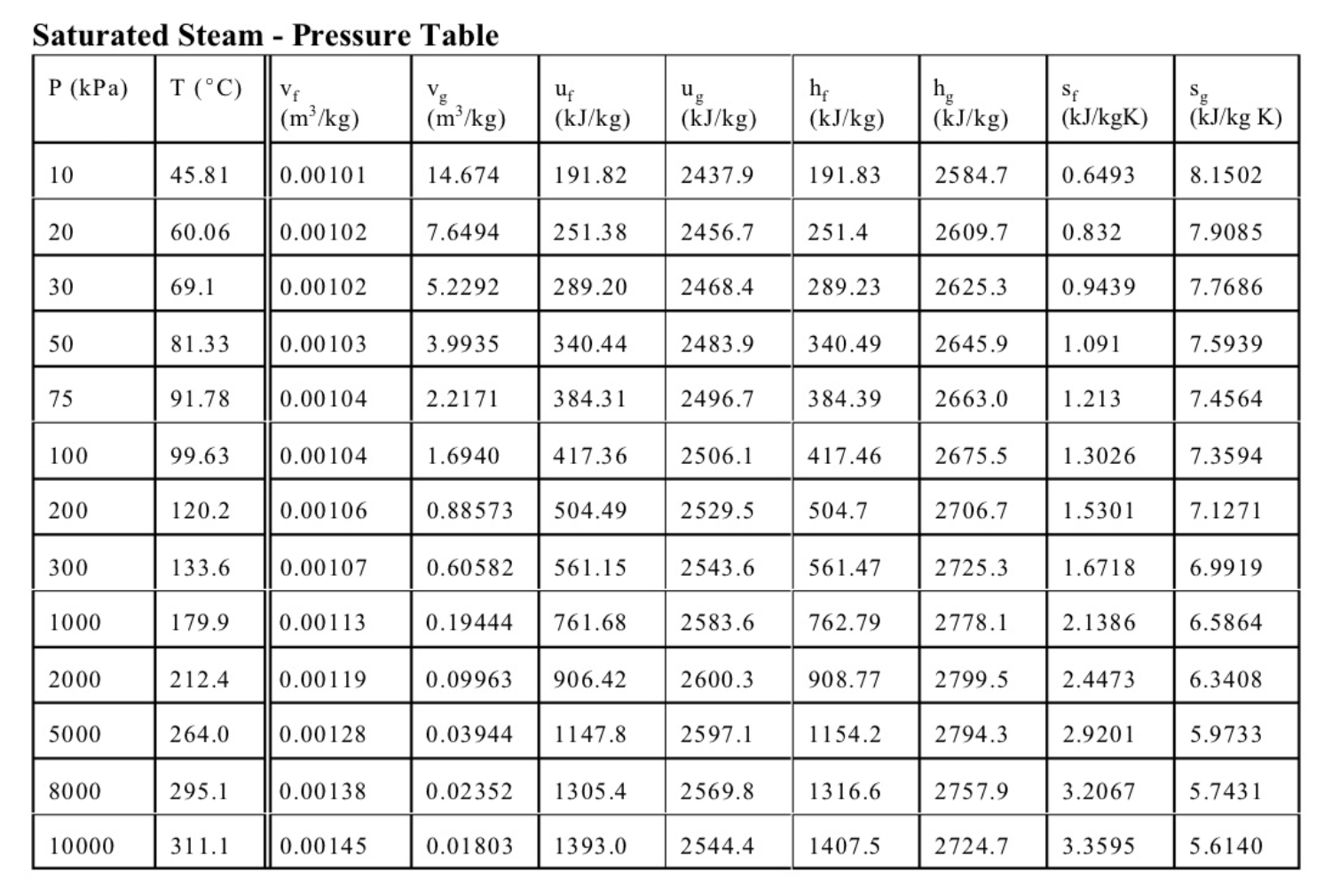
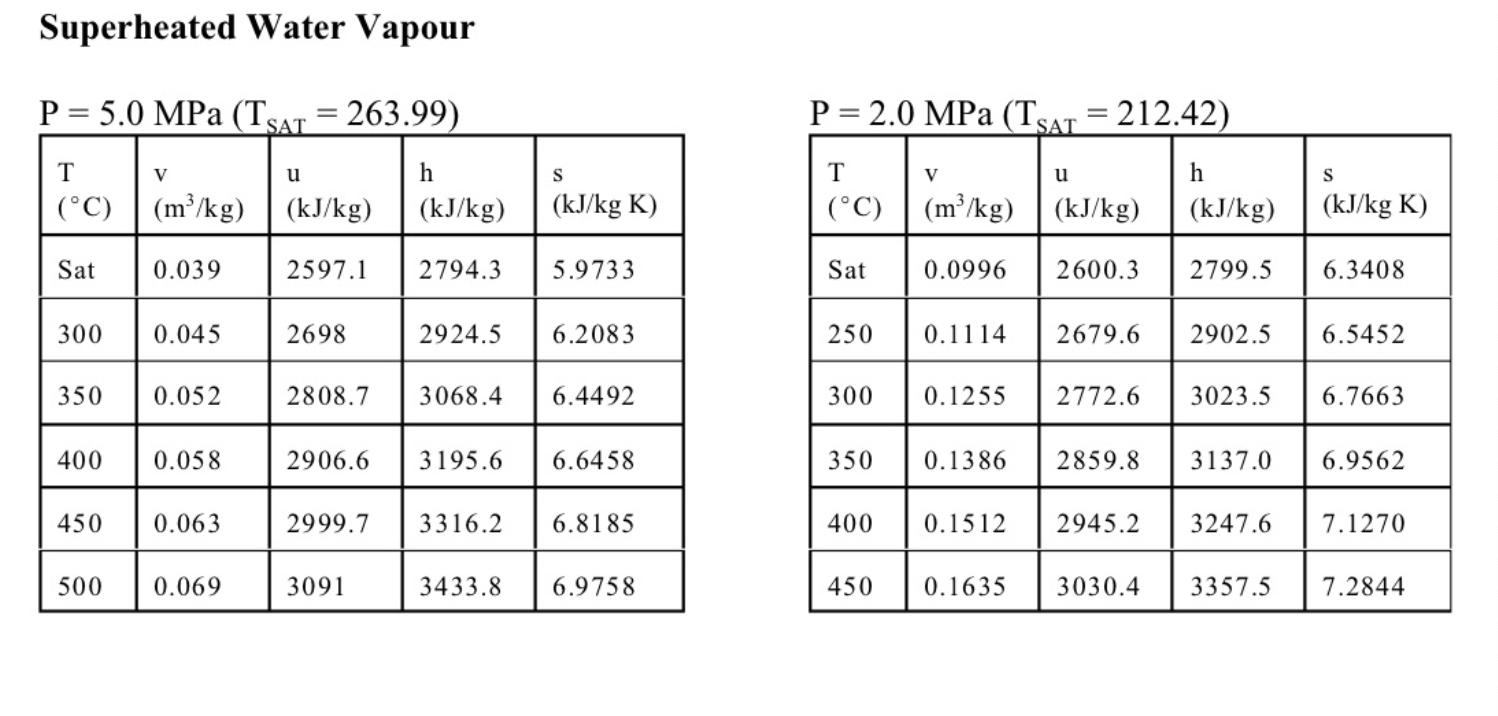
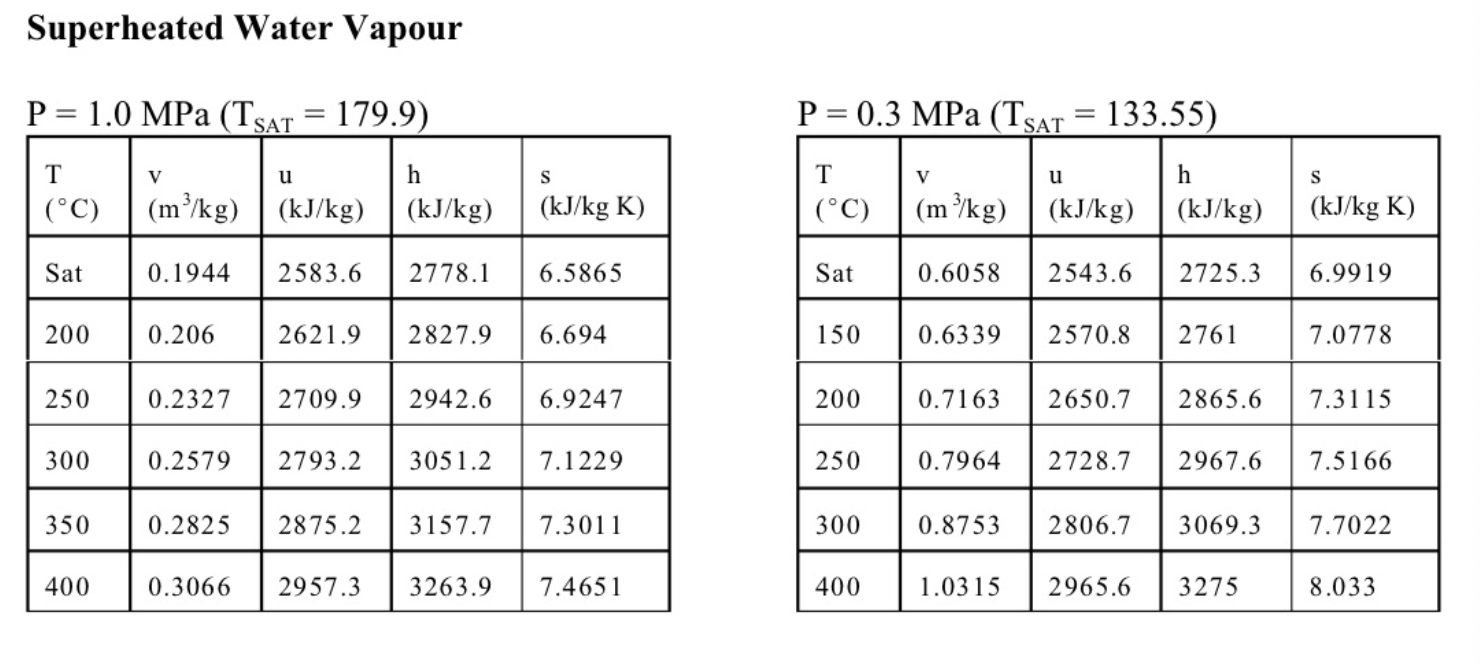
4. (13 marks total) The sketch shows a simple steam power plant. Water enters at point 1 as saturated liquid at T1=20C and is raised to a pressure of P2=2MPa by the pump. It is then turned to steam and raised to a temperature of T3=400C in the boiler, and finally expands in the turbine to a final pressure of P4=100kPa. The turbine and the pump are well insulated and may be assumed to be reversible. (a) (4 marks) Determine the exit state from the turbine (quality or temperature, whichever is appropriate), and the specific work of the turbine in kJ/kg. (b) (4 marks) Calculate the specific work required for the pump in kJ/kg, and the exit temperature from the pump T2. (c) (3 marks) Find the mass flow of water required in kg/s for a turbine work output of 1MW. If the diameter of the pipe leaving the turbine at 4 is 0.1m, determine the velocity of the steam at 4 . (d) (2 mark) Sketch a T-s diagram for these processes, showing the vapour dome. Equations and Other Data Work done in a polytropic process Pvn= constant, control mass: W12=(P2V2P1V1)/(1n) Work done in reversible steady flow: w=12vdP Entropy change for an ideal gas with constant specific heats: s2s1=cPln(T2/T1)Rln(P2/P1) Properties for air: R=0.287kJ/kgK;specificheatsat300K:cP0=1.004kJ/kgK,cv0=0.717kJ/kgK Universal gas constant: R=8.314kJ/kmolK Steam Tables Saturated Steam - Pressure Table \begin{tabular}{|l|l||l|l|l|l|l|l|l|l|} \hline P(kPa) & T(C) & \begin{tabular}{l} vf \\ (m3/kg) \end{tabular} & \begin{tabular}{l} vg \\ (m3/kg) \end{tabular} & \begin{tabular}{l} uf \\ (kJ/kg) \end{tabular} & \begin{tabular}{l} ug \\ (kJ/kg) \end{tabular} & \begin{tabular}{l} hf \\ (kJ/kg) \end{tabular} & \begin{tabular}{l} hg \\ (kJ/kg) \end{tabular} & \begin{tabular}{l} sf \\ (kJ/kgK) \end{tabular} & \begin{tabular}{l} sg \\ (kJ/kgK) \end{tabular} \\ \hline 10 & 45.81 & 0.00101 & 14.674 & 191.82 & 2437.9 & 191.83 & 2584.7 & 0.6493 & 8.1502 \\ \hline 20 & 60.06 & 0.00102 & 7.6494 & 251.38 & 2456.7 & 251.4 & 2609.7 & 0.832 & 7.9085 \\ \hline 30 & 69.1 & 0.00102 & 5.2292 & 289.20 & 2468.4 & 289.23 & 2625.3 & 0.9439 & 7.7686 \\ \hline 50 & 81.33 & 0.00103 & 3.9935 & 340.44 & 2483.9 & 340.49 & 2645.9 & 1.091 & 7.5939 \\ \hline 75 & 91.78 & 0.00104 & 2.2171 & 384.31 & 2496.7 & 384.39 & 2663.0 & 1.213 & 7.4564 \\ \hline 100 & 99.63 & 0.00104 & 1.6940 & 417.36 & 2506.1 & 417.46 & 2675.5 & 1.3026 & 7.3594 \\ \hline 200 & 120.2 & 0.00106 & 0.88573 & 504.49 & 2529.5 & 504.7 & 2706.7 & 1.5301 & 7.1271 \\ \hline 300 & 133.6 & 0.00107 & 0.60582 & 561.15 & 2543.6 & 561.47 & 2725.3 & 1.6718 & 6.9919 \\ \hline 1000 & 179.9 & 0.00113 & 0.19444 & 761.68 & 2583.6 & 762.79 & 2778.1 & 2.1386 & 6.5864 \\ \hline 2000 & 212.4 & 0.00119 & 0.09963 & 906.42 & 2600.3 & 908.77 & 2799.5 & 2.4473 & 6.3408 \\ \hline 5000 & 264.0 & 0.00128 & 0.03944 & 1147.8 & 2597.1 & 1154.2 & 2794.3 & 2.9201 & 5.9733 \\ \hline 8000 & 295.1 & 0.00138 & 0.02352 & 1305.4 & 2569.8 & 1316.6 & 2757.9 & 3.2067 & 5.7431 \\ \hline 10000 & 311.1 & 0.00145 & 0.01803 & 1393.0 & 2544.4 & 1407.5 & 2724.7 & 3.3595 & 5.6140 \\ \hline \end{tabular} Superheated Water Vapour P=5.0MPa(TSAT=263.99) P=2.0MPa(TCT=212.42) Superheated Water Vapour P=1.0MPa(TSAT=179.9) P=0.3MPa(TSAT=133.55)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


