Question
You have carried out the below reaction between an acid chloride with an amine to form an amide. Construct a flow chart to show
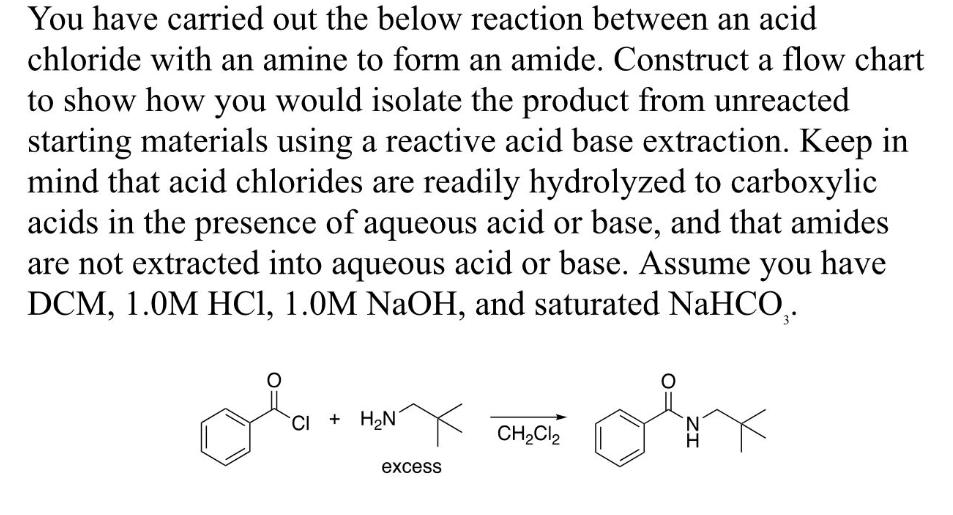
You have carried out the below reaction between an acid chloride with an amine to form an amide. Construct a flow chart to show how you would isolate the product from unreacted starting materials using a reactive acid base extraction. Keep in mind that acid chlorides are readily hydrolyzed to carboxylic acids in the presence of aqueous acid or base, and that amides are not extracted into aqueous acid or base. Assume you have DCM, 1.0M HCl, 1.0M NaOH, and saturated NaHCO. CI+HN N CH2Cl2 excess
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Acidbase extraction is used to form salts of acid chloride and a...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



