Answered step by step
Verified Expert Solution
Question
1 Approved Answer
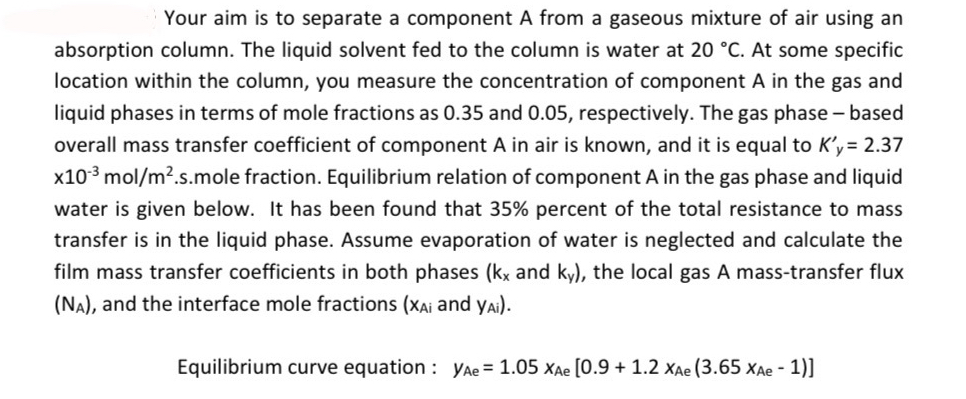
Your aim is to separate a component A from a gaseous mixture of air using an absorption column. The liquid solvent fed to the column
Your aim is to separate a component A from a gaseous mixture of air using an absorption column. The liquid solvent fed to the column is water at At some specific location within the column, you measure the concentration of component in the gas and liquid phases in terms of mole fractions as and respectively. The gas phase based
overall mass transfer coefficient of component in air is known, and it is equal to mole fraction. Equilibrium relation of component in the gas phase and liquid water is given below. It has been found that percent of the total resistance to mass
transfer is in the liquid phase. Assume evaporation of water is neglected and calculate the film mass transfer coefficients in both phases and the local gas A masstransfer flux and the interface mole fractions and : Equilibrium curve equation : can you solve by mass transfer and write assumption this question You don't use any AI systemschatgpt etc.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


